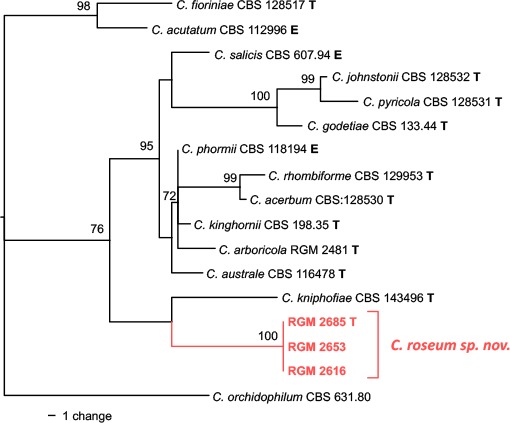
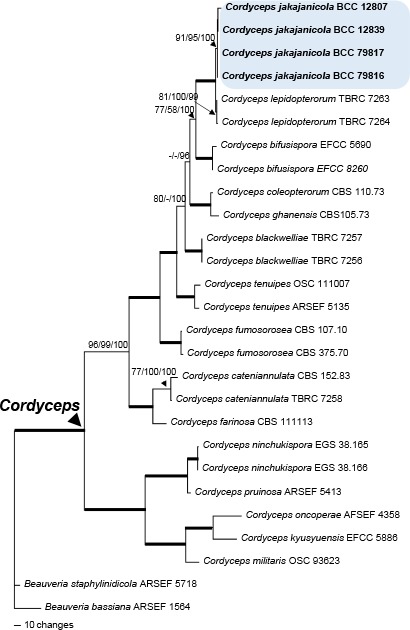
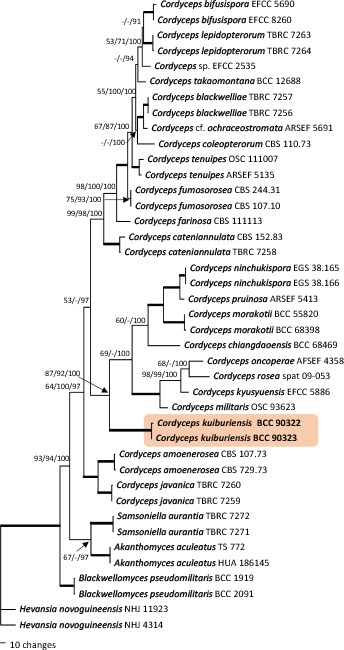
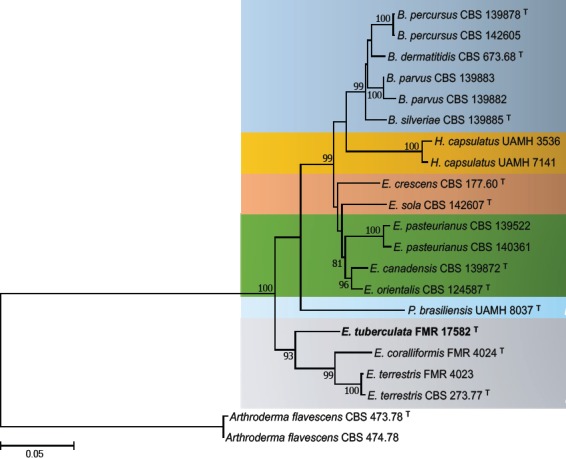
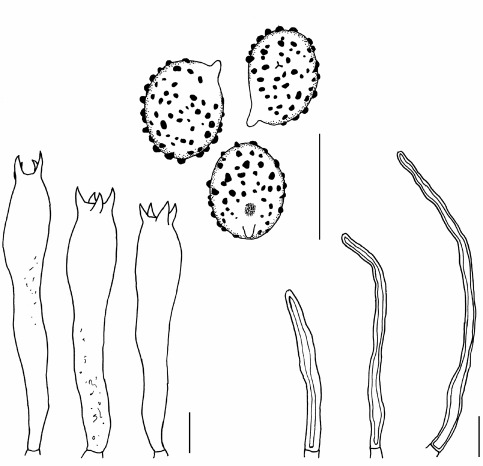
Abstract
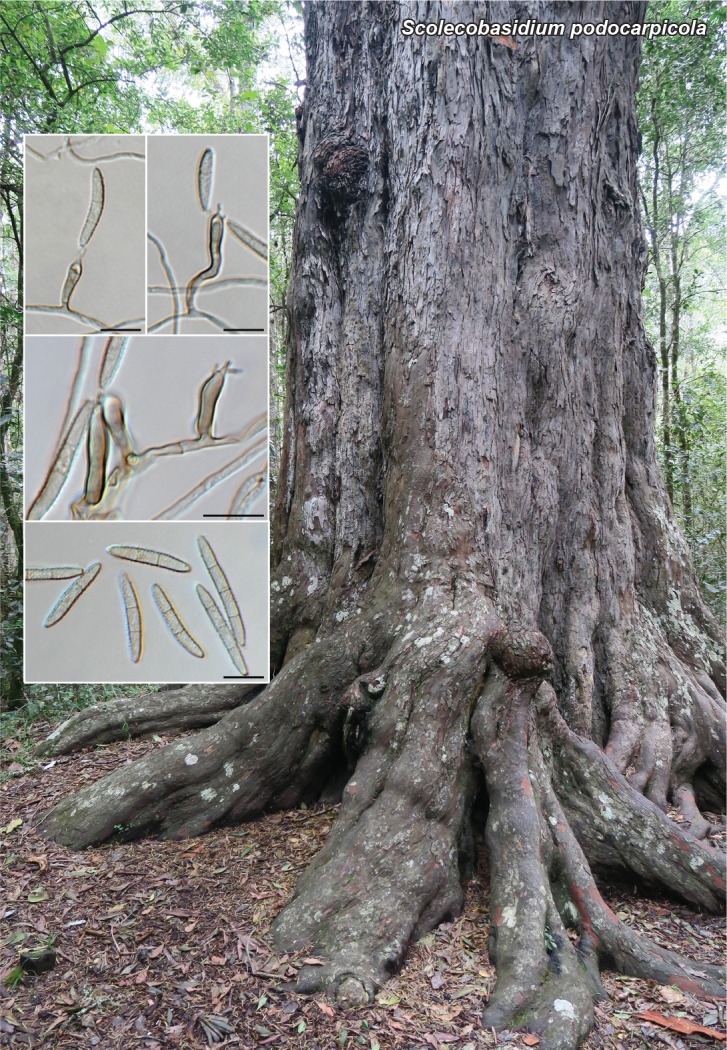
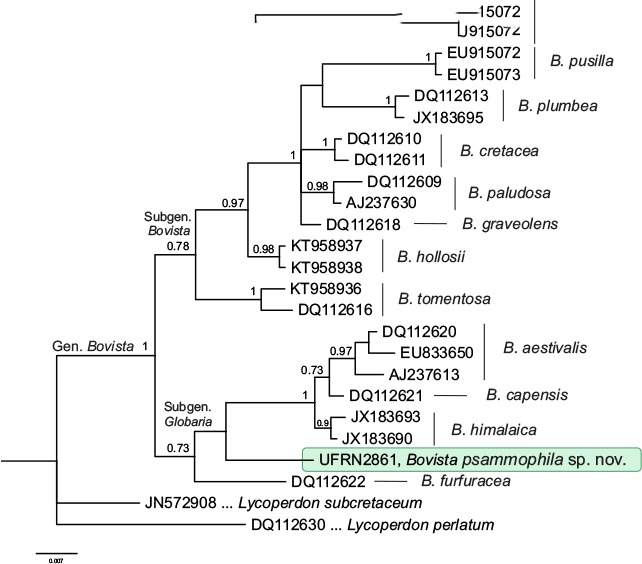
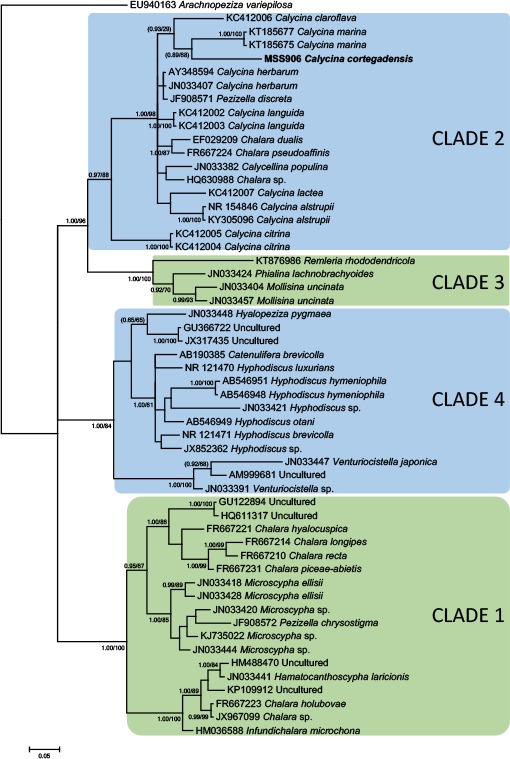
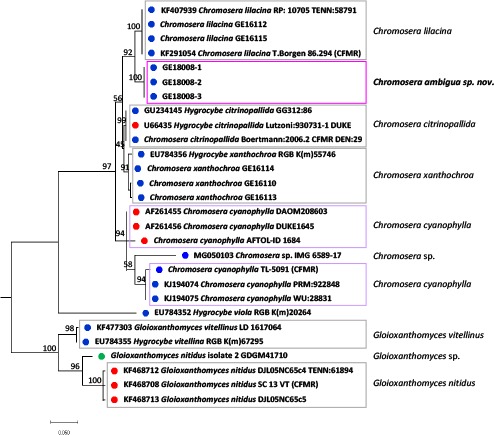
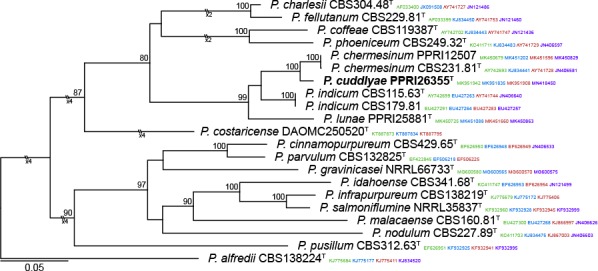
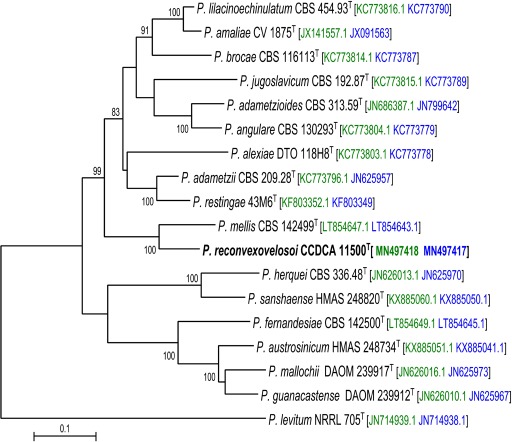
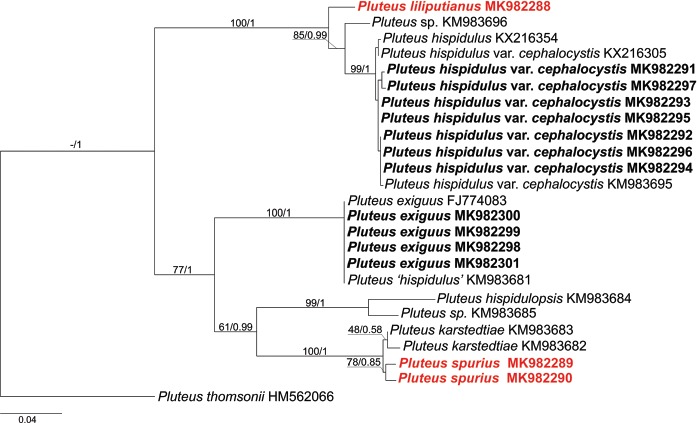
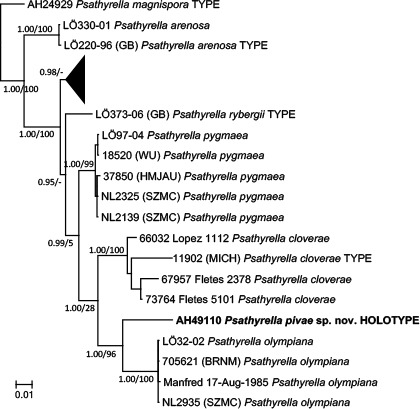
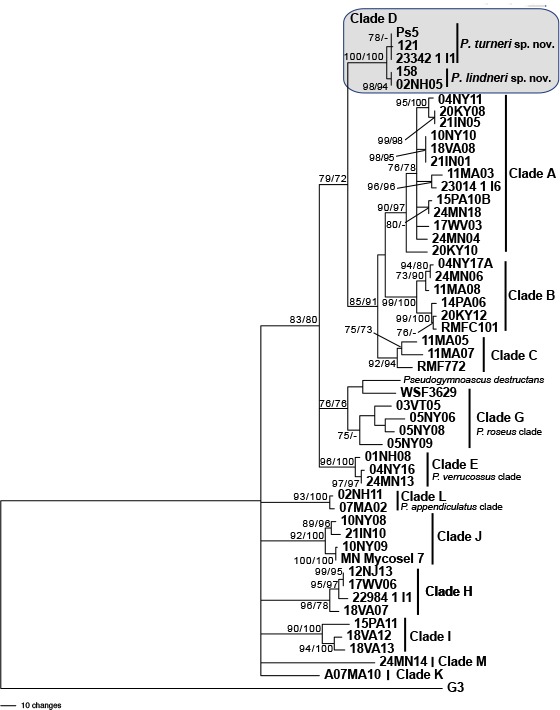
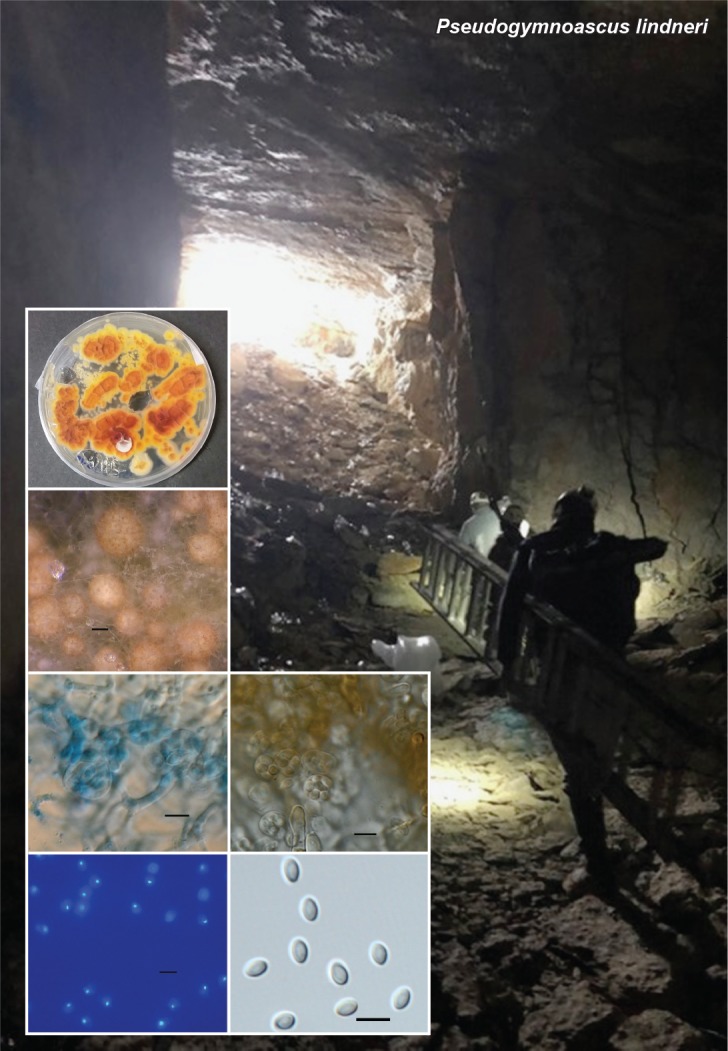
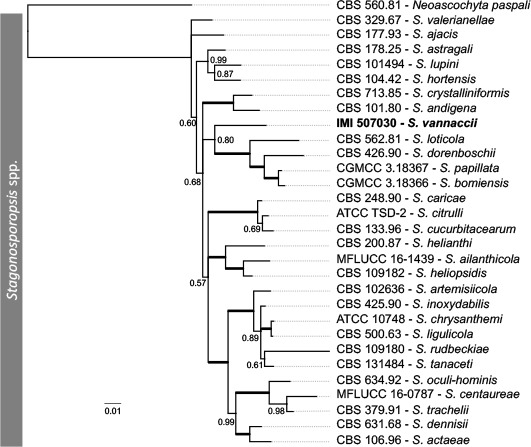
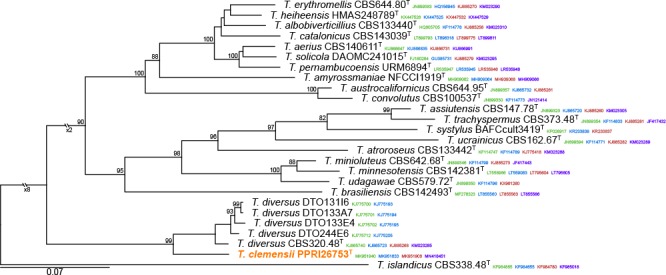
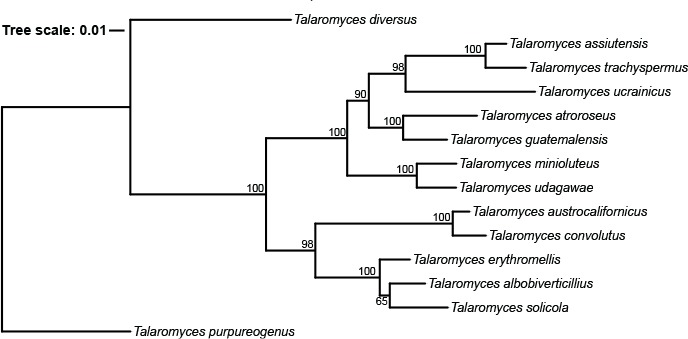
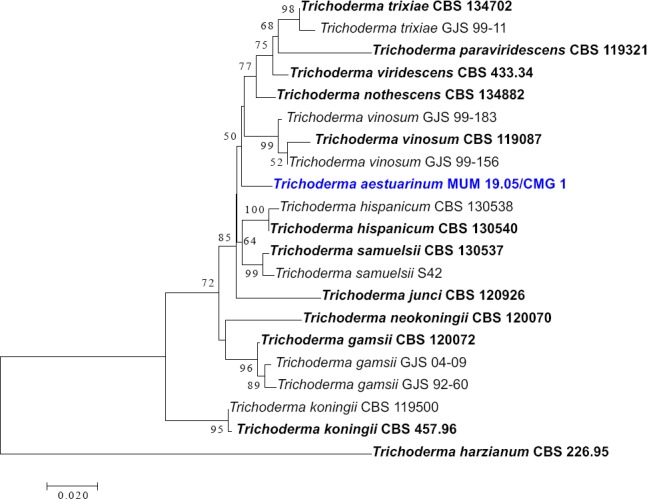
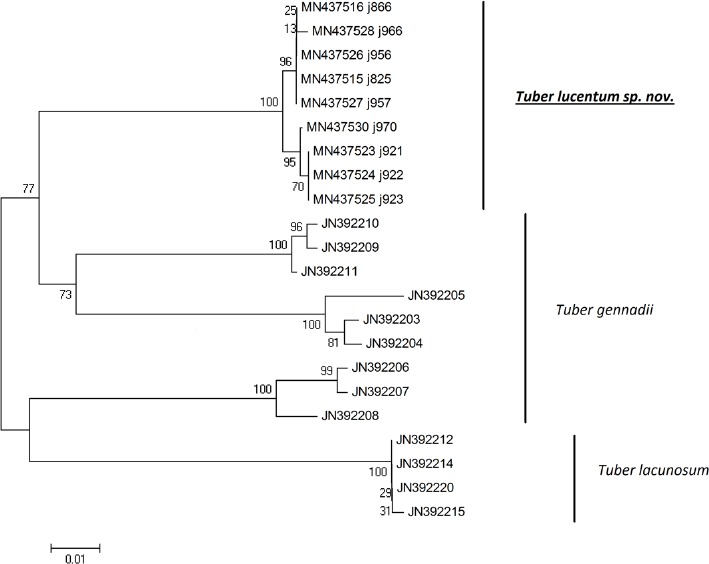
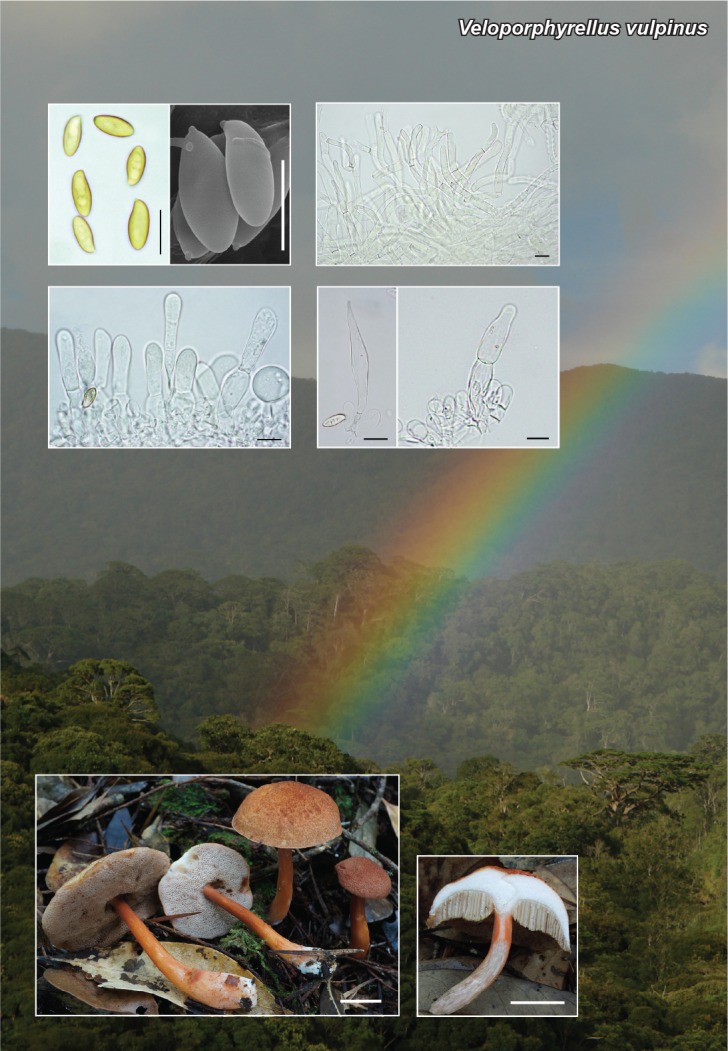
Novel species of fungi described in this study include those from various countries as follows: Antarctica, Apenidiella antarctica from permafrost, Cladosporium fildesense from an unidentified marine sponge. Argentina, Geastrum wrightii on humus in mixed forest. Australia, Golovinomyces glandulariae on Glandularia aristigera, Neoanungitea eucalyptorum on leaves of Eucalyptus grandis, Teratosphaeria corymbiicola on leaves of Corymbia ficifolia, Xylaria eucalypti on leaves of Eucalyptus radiata. Brazil, Bovista psammophila on soil, Fusarium awaxy on rotten stalks of Zea mays, Geastrum lanuginosum on leaf litter covered soil, Hermetothecium mikaniae-micranthae (incl. Hermetothecium gen. nov.) on Mikania micrantha, Penicillium reconvexovelosoi in soil, Stagonosporopsis vannaccii from pod of Glycine max. British Virgin Isles, Lactifluus guanensis on soil. Canada, Sorocybe oblongispora on resin of Picea rubens. Chile, Colletotrichum roseum on leaves of Lapageria rosea. China, Setophoma caverna from carbonatite in Karst cave. Colombia, Lareunionomyces eucalypticola on leaves of Eucalyptus grandis. Costa Rica, Psathyrella pivae on wood. Cyprus, Clavulina iris on calcareous substrate. France, Chromosera ambigua and Clavulina iris var. occidentalis on soil. French West Indies, Helminthosphaeria hispidissima on dead wood. Guatemala, Talaromyces guatemalensis in soil. Malaysia, Neotracylla pini (incl. Tracyllales ord. nov. and Neotracylla gen. nov.) and Vermiculariopsiella pini on needles of Pinus tecunumanii. New Zealand, Neoconiothyrium viticola on stems of Vitis vinifera, Parafenestella pittospori on Pittosporum tenuifolium, Pilidium novae-zelandiae on Phoenix sp. Pakistan, Russula quercus-floribundae on forest floor. Portugal, Trichoderma aestuarinum from saline water. Russia, Pluteus liliputianus on fallen branch of deciduous tree, Pluteus spurius on decaying deciduous wood or soil. South Africa, Alloconiothyrium encephalarti, Phyllosticta encephalarticola and Neothyrostroma encephalarti (incl. Neothyrostroma gen. nov.) on leaves of Encephalartos sp., Chalara eucalypticola on leaf spots of Eucalyptus grandis × urophylla, Clypeosphaeria oleae on leaves of Olea capensis, Cylindrocladiella postalofficium on leaf litter of Sideroxylon inerme, Cylindromonium eugeniicola (incl. Cylindromonium gen. nov.) on leaf litter of Eugenia capensis, Cyphellophora goniomatis on leaves of Gonioma kamassi, Nothodactylaria nephrolepidis (incl. Nothodactylaria gen. nov. and Nothodactylariaceae fam. nov.) on leaves of Nephrolepis exaltata, Falcocladium eucalypti and Gyrothrix eucalypti on leaves of Eucalyptus sp., Gyrothrix oleae on leaves of Olea capensis subsp. macrocarpa, Harzia metrosideri on leaf litter of Metrosideros sp., Hippopotamyces phragmitis (incl. Hippopotamyces gen. nov.) on leaves of Phragmites australis, Lectera philenopterae on Philenoptera violacea, Leptosillia mayteni on leaves of Maytenus heterophylla, Lithohypha aloicola and Neoplatysporoides aloes on leaves of Aloe sp., Millesimomyces rhoicissi (incl. Millesimomyces gen. nov.) on leaves of Rhoicissus digitata, Neodevriesia strelitziicola on leaf litter of Strelitzia nicolai, Neokirramyces syzygii (incl. Neokirramyces gen. nov.) on leaf spots of Syzygium sp., Nothoramichloridium perseae (incl. Nothoramichloridium gen. nov. and Anungitiomycetaceae fam. nov.) on leaves of Persea americana, Paramycosphaerella watsoniae on leaf spots of Watsonia sp., Penicillium cuddlyae from dog food, Podocarpomyces knysnanus (incl. Podocarpomyces gen. nov.) on leaves of Podocarpus falcatus, Pseudocercospora heteropyxidicola on leaf spots of Heteropyxis natalensis, Pseudopenidiella podocarpi, Scolecobasidium podocarpi and Ceramothyrium podocarpicola on leaves of Podocarpus latifolius, Scolecobasidium blechni on leaves of Blechnum capense, Stomiopeltis syzygii on leaves of Syzygium chordatum, Strelitziomyces knysnanus (incl. Strelitziomyces gen. nov.) on leaves of Strelitzia alba, Talaromyces clemensii from rotting wood in goldmine, Verrucocladosporium visseri on Carpobrotus edulis. Spain, Boletopsis mediterraneensis on soil, Calycina cortegadensisi on a living twig of Castanea sativa, Emmonsiellopsis tuberculata in fluvial sediments, Mollisia cortegadensis on dead attached twig of Quercus robur, Psathyrella ovispora on soil, Pseudobeltrania lauri on leaf litter of Laurus azorica, Terfezia dunensis in soil, Tuber lucentum in soil, Venturia submersa on submerged plant debris. Thailand, Cordyceps jakajanicola on cicada nymph, Cordyceps kuiburiensis on spider, Distoseptispora caricis on leaves of Carex sp., Ophiocordyceps khonkaenensis on cicada nymph. USA, Cytosporella juncicola and Davidiellomyces juncicola on culms of Juncus effusus, Monochaetia massachusettsianum from air sample, Neohelicomyces melaleucae and Periconia neobrittanica on leaves of Melaleuca styphelioides × lanceolata, Pseudocamarosporium eucalypti on leaves of Eucalyptus sp., Pseudogymnoascus lindneri from sediment in a mine, Pseudogymnoascus turneri from sediment in a railroad tunnel, Pulchroboletus sclerotiorum on soil, Zygosporium pseudomasonii on leaf of Serenoa repens. Vietnam, Boletus candidissimus and Veloporphyrellus vulpinus on soil. Morphological and culture characteristics are supported by DNA barcodes.
Keywords: ITS nrDNA barcodes, LSU, new taxa, systematics
Overview Agaricomycetes (Basidiomycota) phylogeny – part 1.
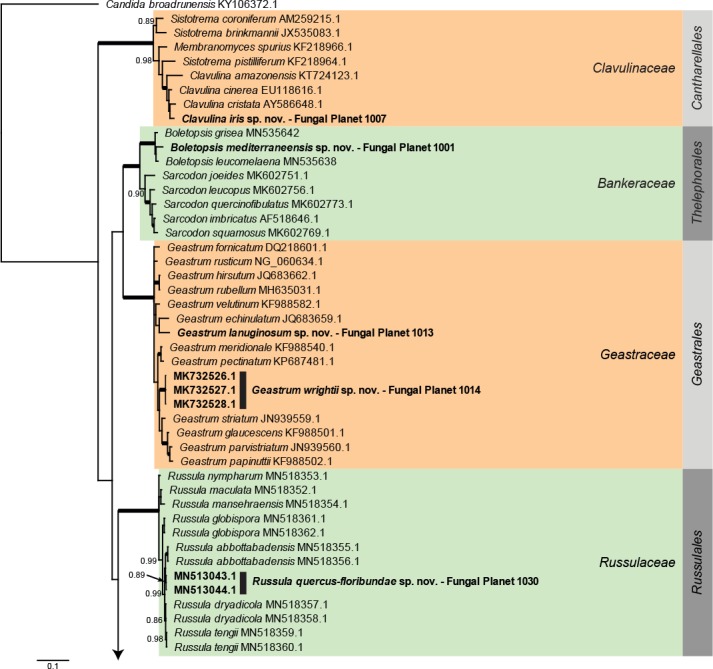
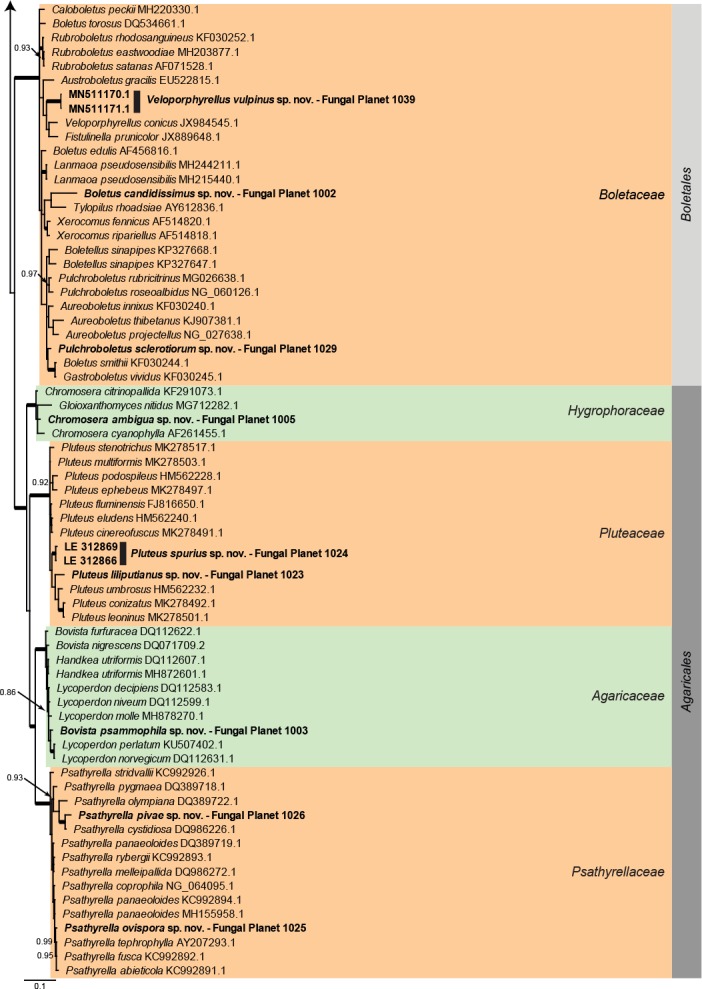
Consensus phylogram (50 % majority rule) of 3 602 trees resulting from a Bayesian analysis of the LSU sequence alignment (115 sequences including outgroup; 764 aligned positions; 427 unique site patterns) using MrBayes v. 3.2.6 (Ronquist et al. 2012). Bayesian posterior probabilities (PP) > 0.84 are shown at the nodes and thickened lines represent nodes with PP = 1.00. The scale bar represents the expected changes per site. Families and orders are indicated with coloured blocks to the right of the tree. GenBank accession and/or Fungal Planet numbers are indicated behind the species names. The tree was rooted to Candida broadrunensis (GenBank KY106372.1) and the taxonomic novelties described in this study for which LSU sequence data were available are indicated in bold face. The alignment and tree were deposited in TreeBASE (Submission ID S25229).
Overview Dothideomycetes phylogeny – part 1.
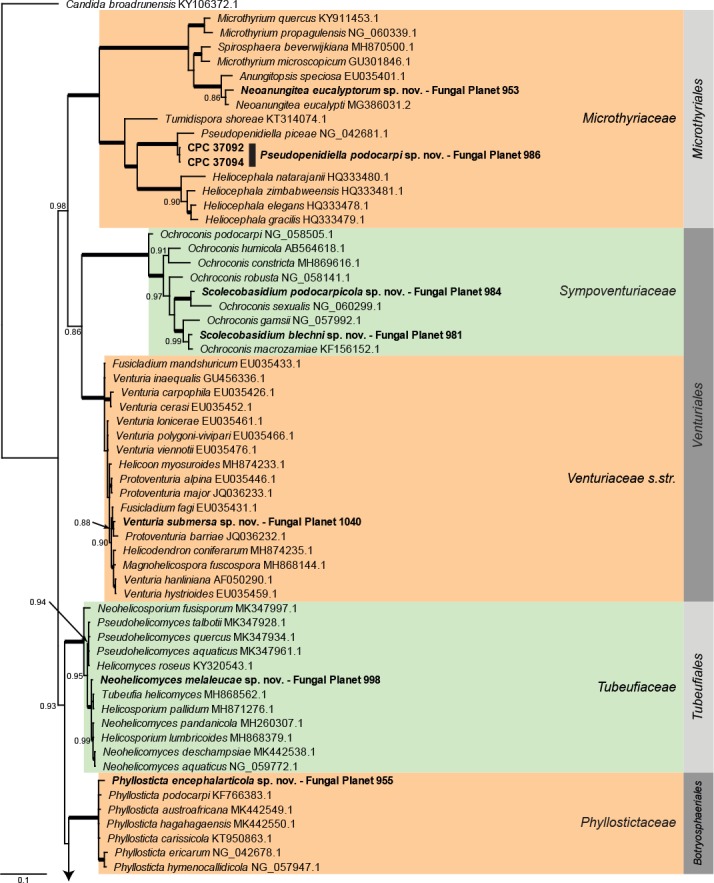
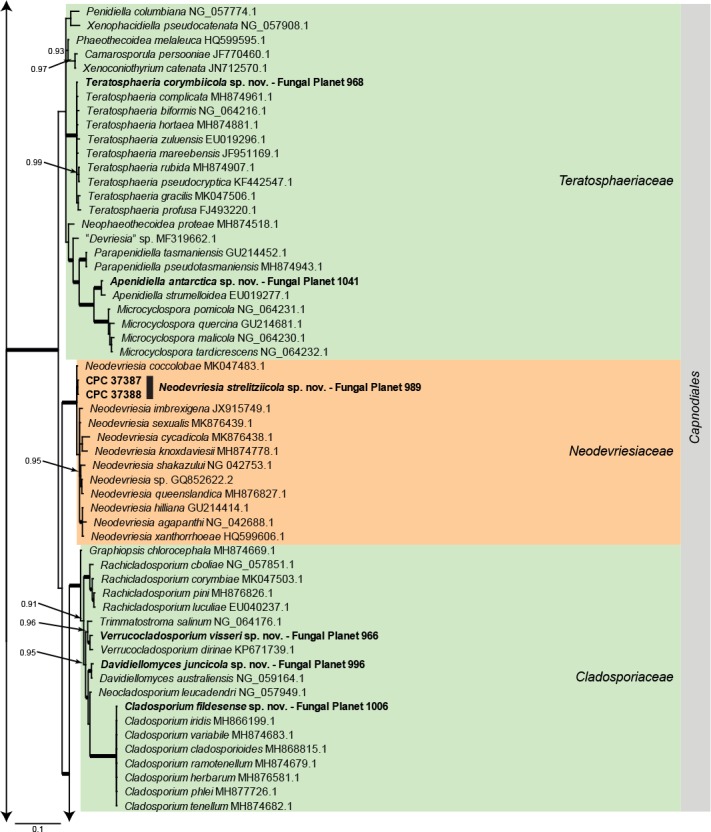
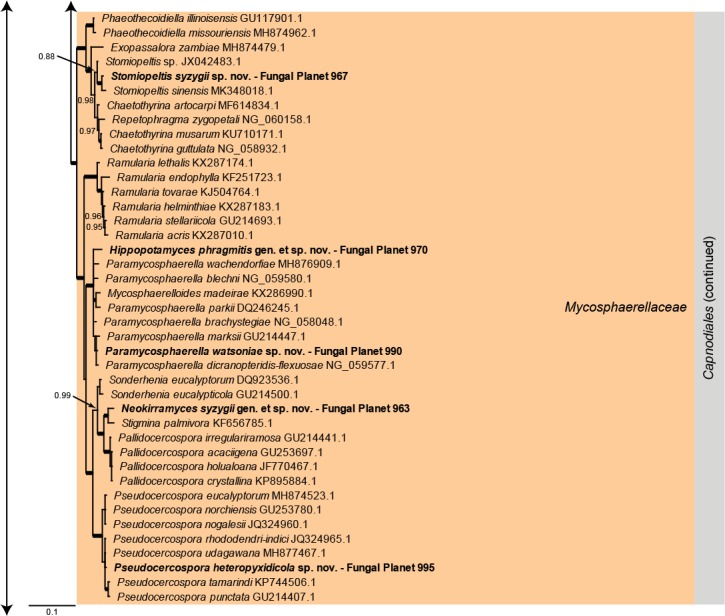
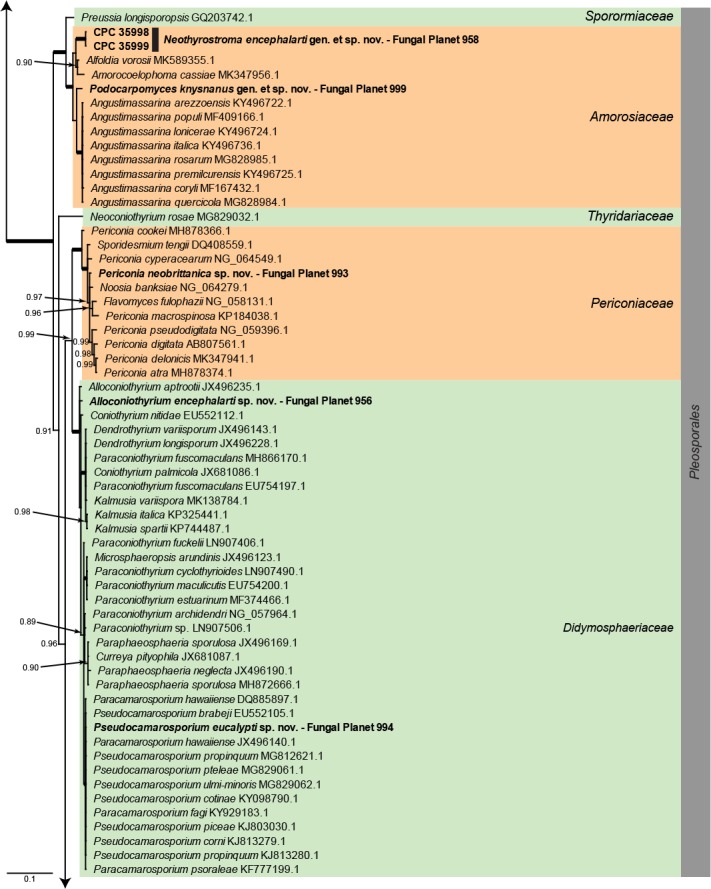
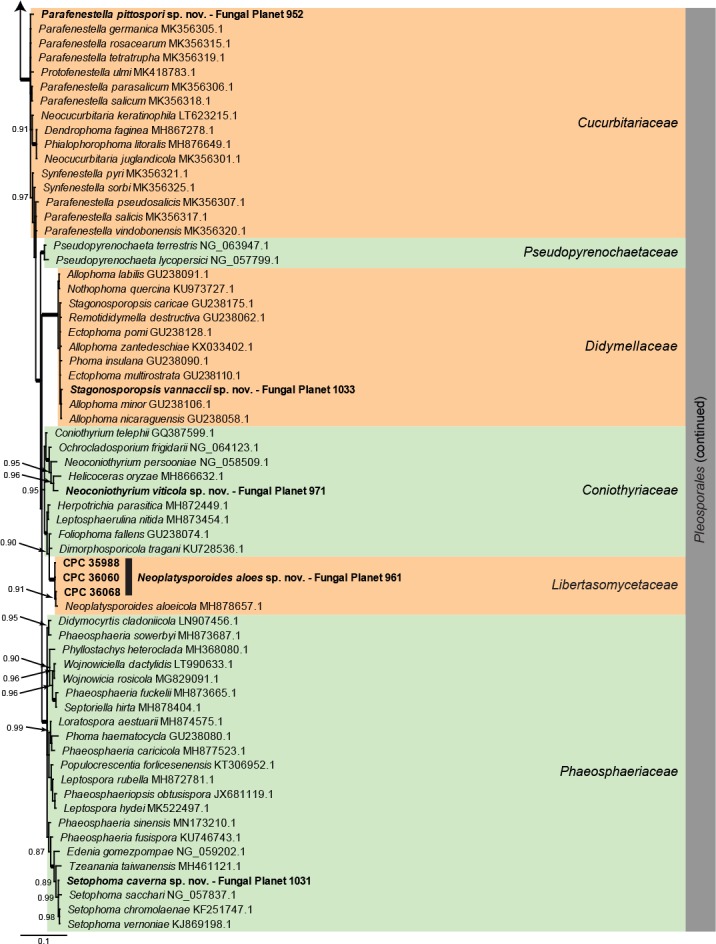
Consensus phylogram (50 % majority rule) of 80 252 trees resulting from a Bayesian analysis of the LSU sequence alignment (284 sequences including outgroup; 797 aligned positions; 431 unique site patterns) using MrBayes v. 3.2.6 (Ronquist et al. 2012). Bayesian posterior probabilities (PP) > 0.84 are shown at the nodes and thickened lines represent nodes with PP = 1.00. The scale bar represents the expected changes per site. Families and orders are indicated with coloured blocks to the right of the tree. GenBank accession and/or Fungal Planet numbers are indicated behind the species names. The tree was rooted to Candida broadrunensis (GenBank KY106372.1) and the taxonomic novelties described in this study for which LSU sequence data were available are indicated in bold face. The alignment and tree were deposited in TreeBASE (Submission ID S25229).
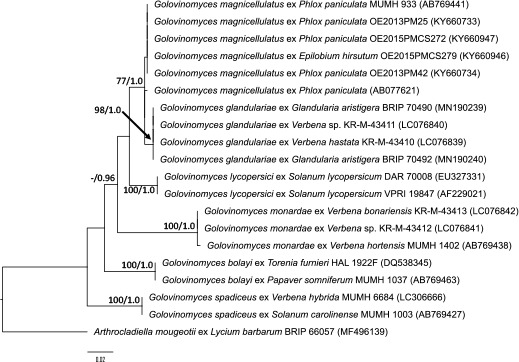
Overview Eurotiomycetes phylogeny.
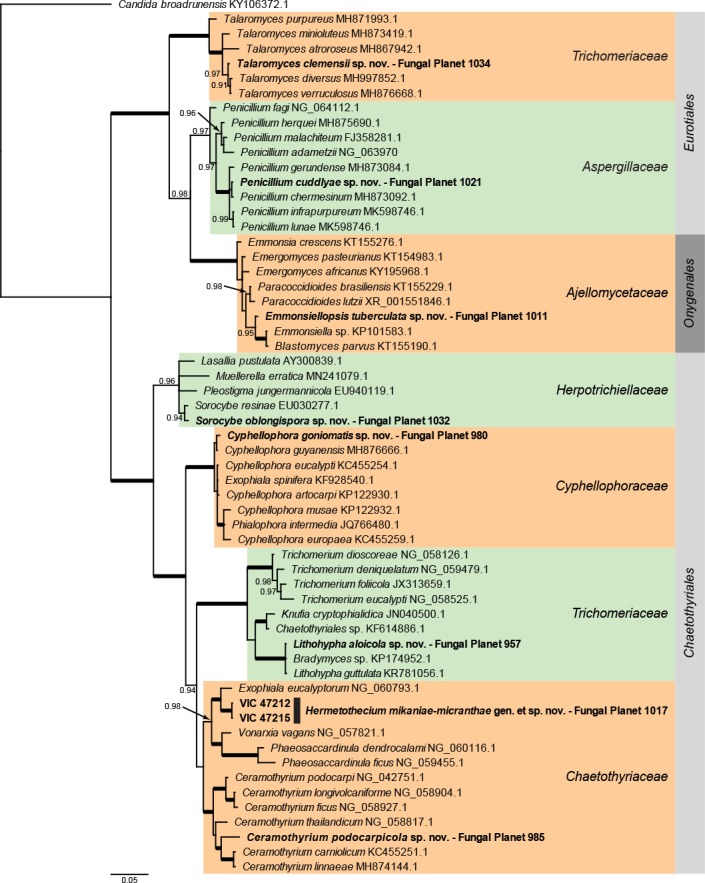
Consensus phylogram (50 % majority rule) of 9 002 trees resulting from a Bayesian analysis of the LSU sequence alignment (59 sequences including outgroup; 796 aligned positions; 308 unique site patterns) using MrBayes v. 3.2.6 (Ronquist et al. 2012). Bayesian posterior probabilities (PP) > 0.84 are shown at the nodes and thickened lines represent nodes with PP = 1.00. The scale bar represents the expected changes per site. Families and orders are indicated with coloured blocks to the right of the tree. GenBank accession and/or Fungal Planet numbers are indicated behind the species names. The tree was rooted to Candida broadrunensis (GenBank KY106372.1) and the taxonomic novelties described in this study for which LSU sequence data were available are indicated in bold face. The alignment and tree were deposited in TreeBASE (Submission ID S25229).
Overview Lecanoromycetes and Pezizomycetes phylogeny.
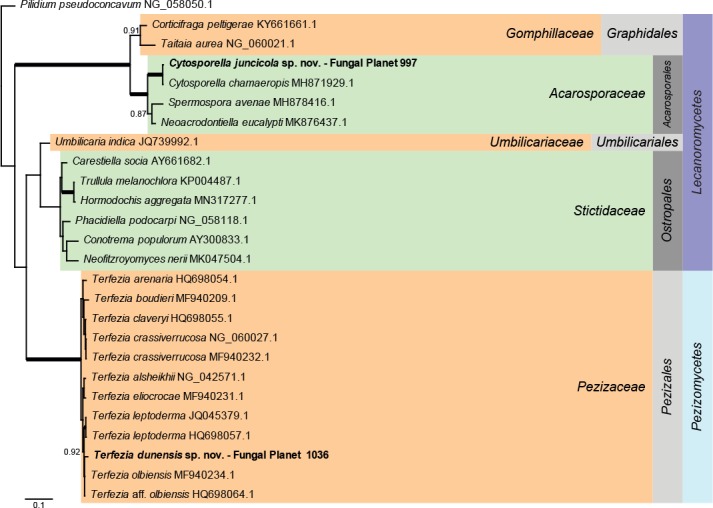
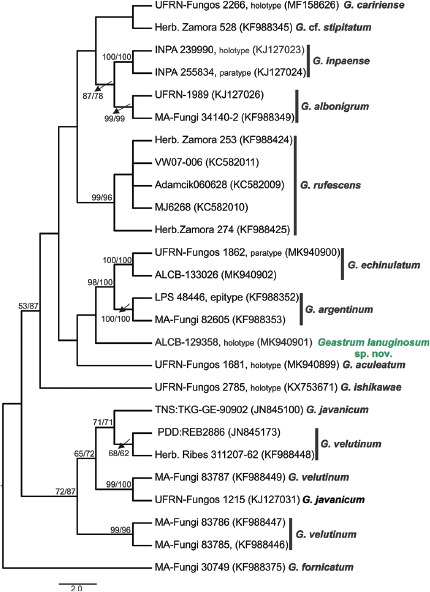
Consensus phylogram (50 % majority rule) of 3 002 trees resulting from a Bayesian analysis of the LSU sequence alignment (26 sequences including outgroup; 760 aligned positions; 264 unique site patterns) using MrBayes v. 3.2.6 (Ronquist et al. 2012). Bayesian posterior probabilities (PP) > 0.84 are shown at the nodes and thickened lines represent nodes with PP = 1.00. The scale bar represents the expected changes per site. Families, orders and classes are indicated with coloured blocks to the right of the tree. GenBank accession or Fungal Planet numbers are indicated behind the species names. The tree was rooted to Pilidium pseudoconcavum (GenBank NG_058050.1) and the taxonomic novelties described in this study for which LSU sequence data were available are indicated in bold face. The alignment and tree were deposited in TreeBASE (Submission ID S25229).
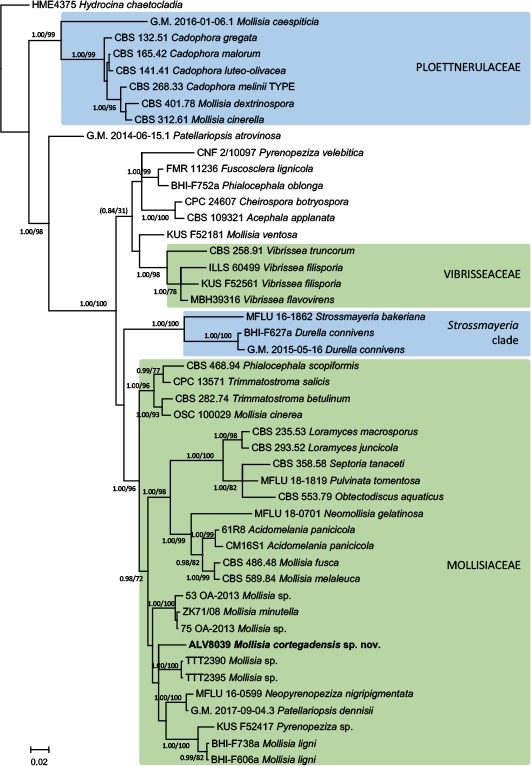
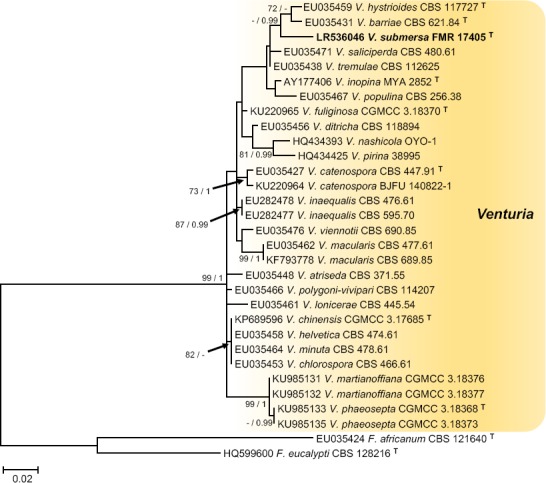
Overview Leotiomycetes phylogeny.
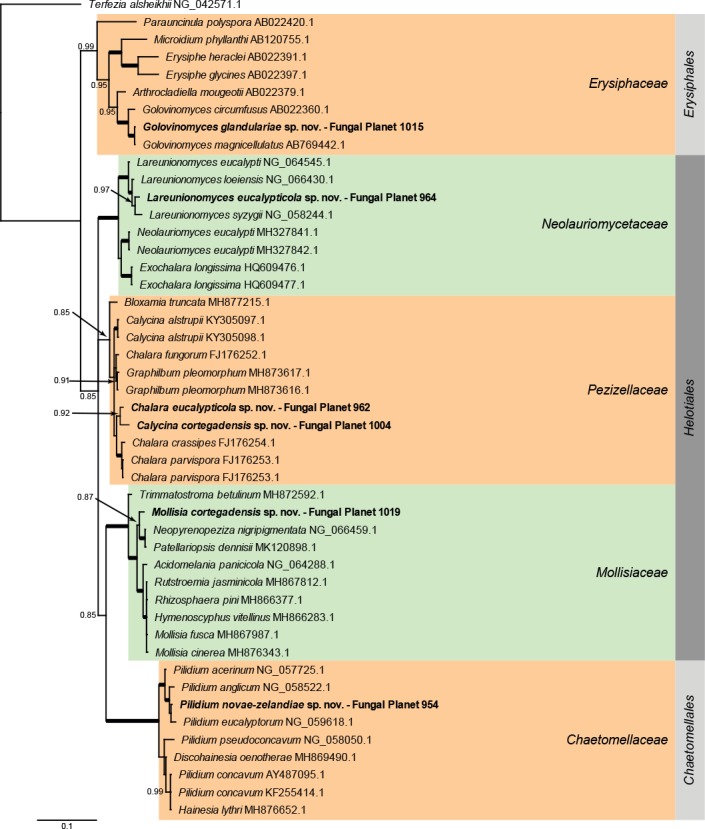
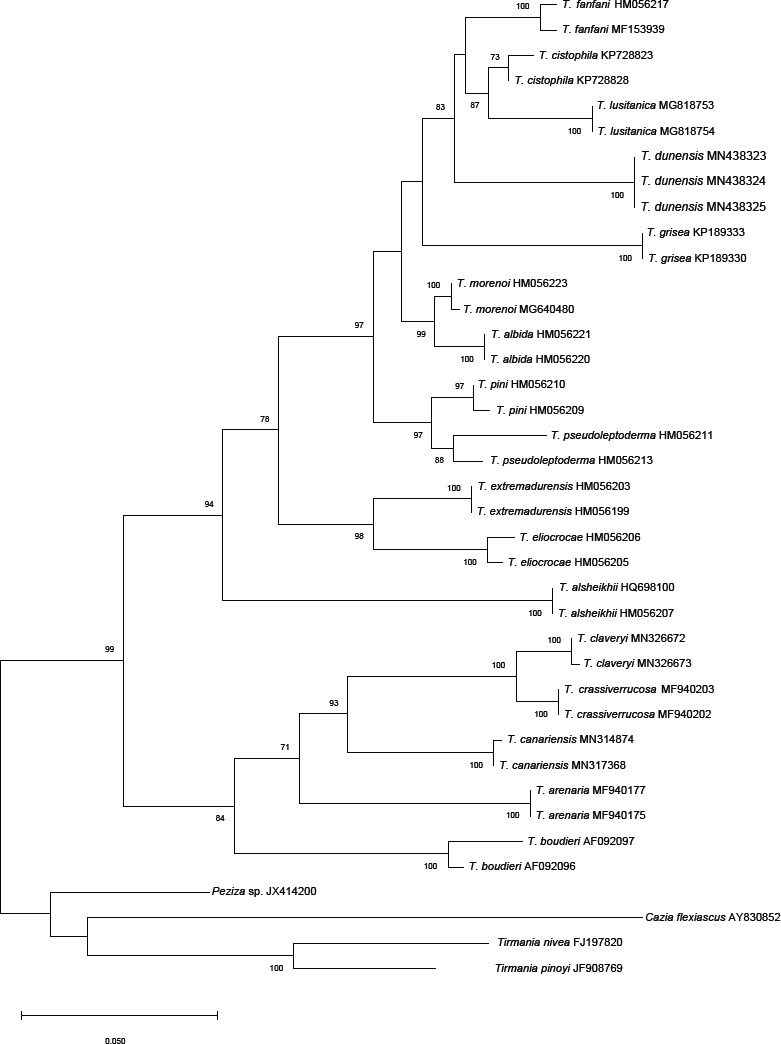
Consensus phylogram (50 % majority rule) of 3 752 trees resulting from a Bayesian analysis of the LSU sequence alignment (47 sequences including outgroup; 752 aligned positions; 199 unique site patterns) using MrBayes v. 3.2.6 (Ronquist et al. 2012). Bayesian posterior probabilities (PP) > 0.84 are shown at the nodes and thickened lines represent nodes with PP = 1.00. The scale bar represents the expected changes per site. Families and orders are indicated with coloured blocks to the right of the tree. GenBank accession or Fungal Planet numbers are indicated behind the species names. The tree was rooted to Terfezia alsheikhii (GenBank NG_042571.1) and the taxonomic novelties described in this study for which LSU sequence data were available are indicated in bold face. The alignment and tree were deposited in TreeBASE (Submission ID S25229).
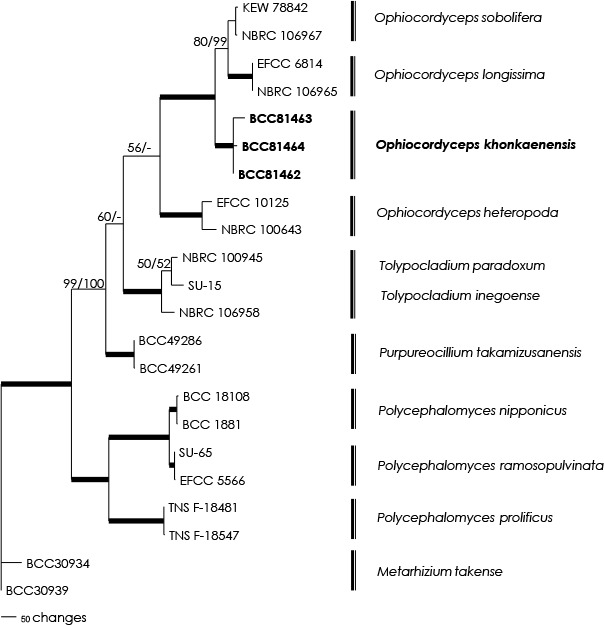
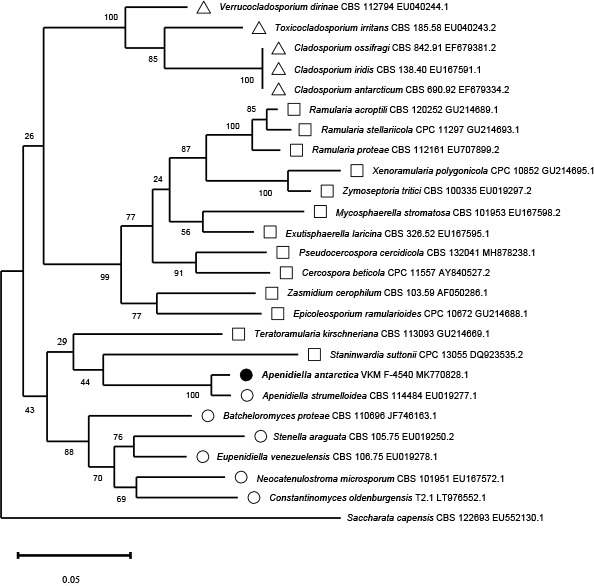
Overview Cordycipitaceae and Ophiocordycipitaceae (Hypocreales, Sordariomycetes) phylogeny.
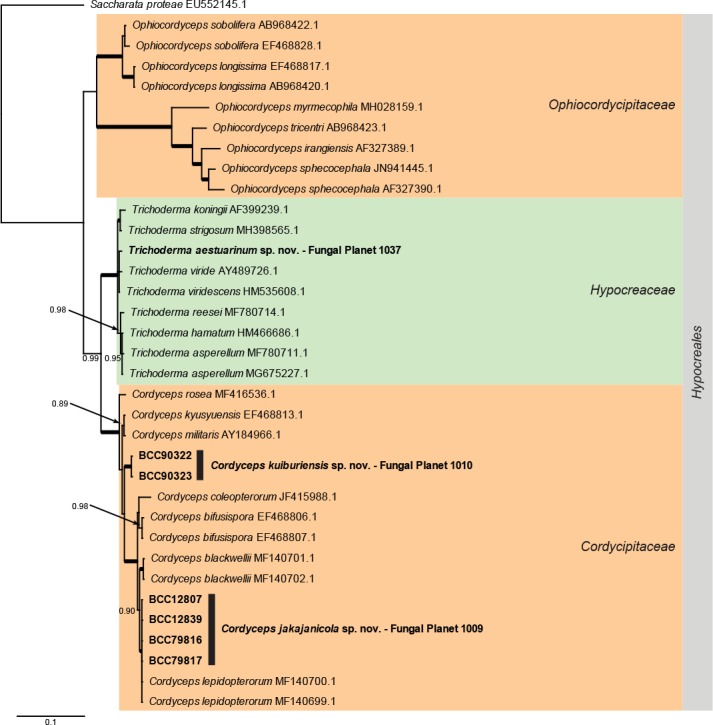
Consensus phylogram (50 % majority rule) of 1 502 trees resulting from a Bayesian analysis of the LSU sequence alignment (35 sequences including outgroup; 798 aligned positions; 242 unique site patterns) using MrBayes v. 3.2.6 (Ronquist et al. 2012). Bayesian posterior probabilities (PP) > 0.84 are shown at the nodes and thickened lines represent nodes with PP = 1.00. The scale bar represents the expected changes per site. Families and orders are indicated with coloured blocks to the right of the tree. GenBank accession and/or Fungal Planet numbers are indicated behind the species names. The tree was rooted to Saccharata proteae (GenBank EU552145.1) and the taxonomic novelties described in this study for which LSU sequence data were available are indicated in bold face. The alignment and tree were deposited in TreeBASE (Submission ID S25229).
Overview Nectriaceae (Hypocreales, Sordariomycetes) phylogeny.
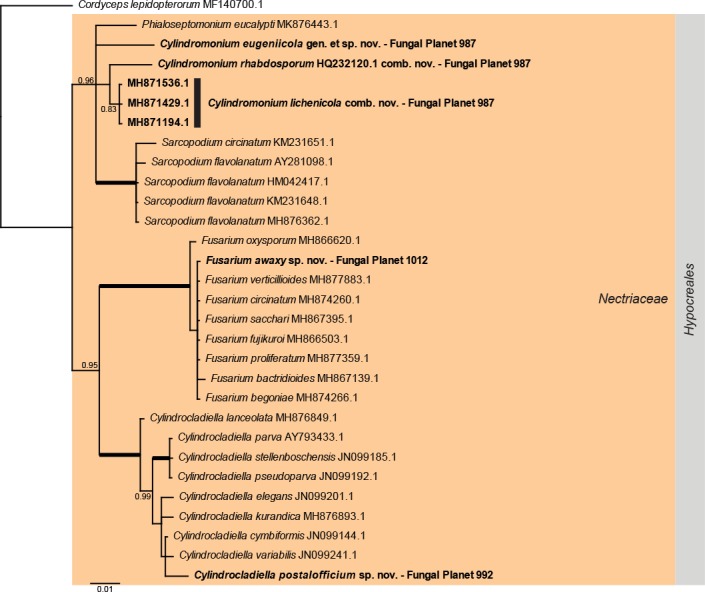
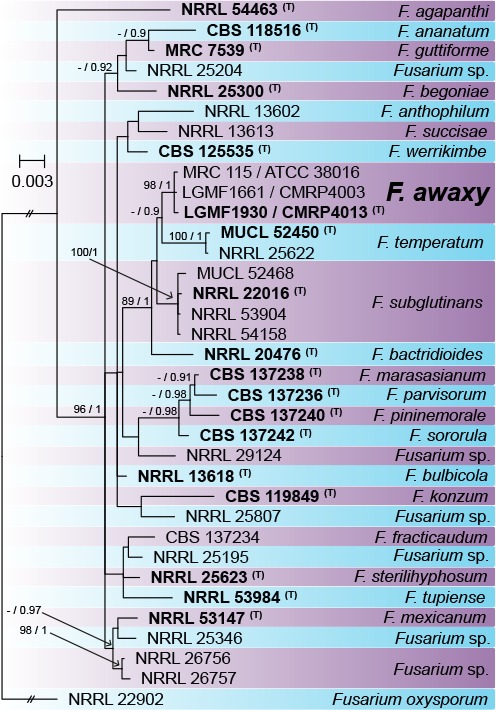
Consensus phylogram (50 % majority rule) of 2 252 trees resulting from a Bayesian analysis of the LSU sequence alignment (30 sequences including outgroup; 778 aligned positions; 95 unique site patterns) using MrBayes v. 3.2.6 (Ronquist et al. 2012). Bayesian posterior probabilities (PP) > 0.84 are shown at the nodes and thickened lines represent nodes with PP = 1.00. The scale bar represents the expected changes per site. The family and order are indicated with coloured blocks to the right of the tree. GenBank accession and/or Fungal Planet numbers are indicated behind the species names. The tree was rooted to Cordyceps lepidopterorum (GenBank MF140700.1) and the taxonomic novelties described in this study for which LSU sequence data were available are indicated in bold face. The alignment and tree were deposited in TreeBASE (Submission ID S25229).
Overview other orders (Sordariomycetes) phylogeny – part 1.
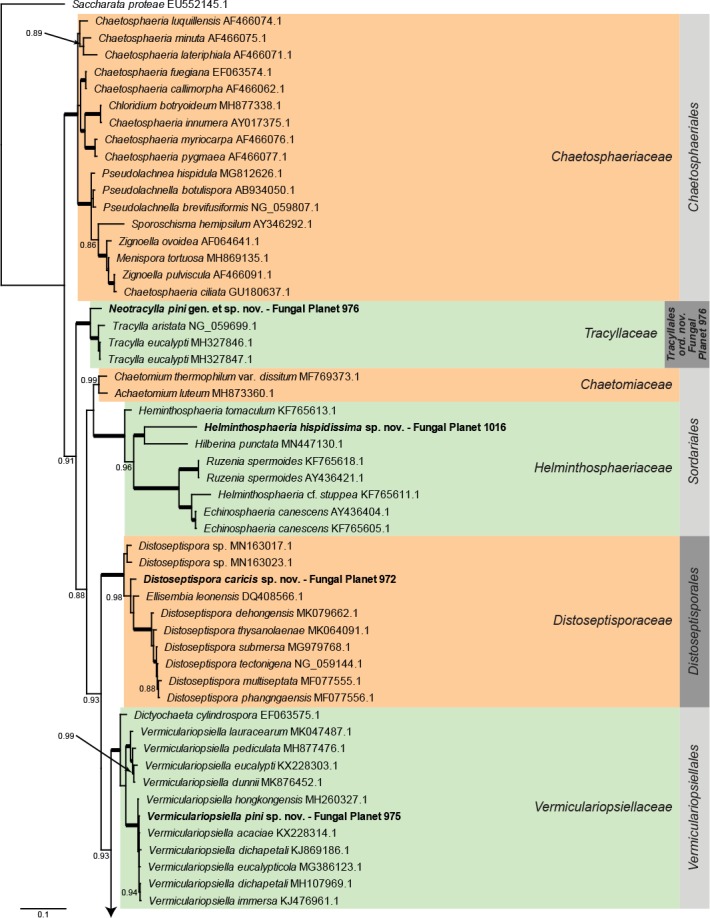
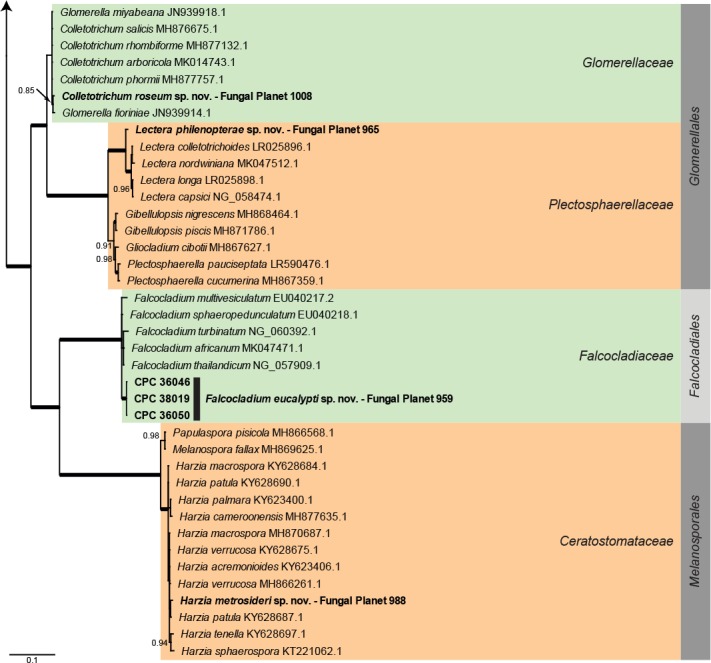
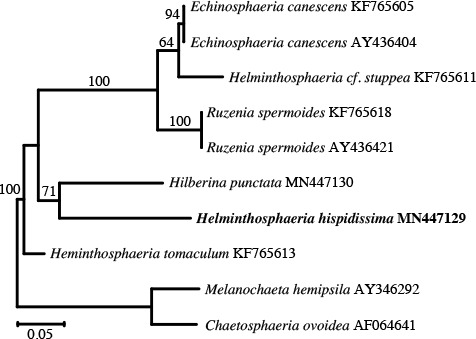
Consensus phylogram (50 % majority rule) of 6 002 trees resulting from a Bayesian analysis of the LSU sequence alignment (93 sequences including outgroup; 825 aligned positions; 405 unique site patterns) using MrBayes v. 3.2.6 (Ronquist et al. 2012). Bayesian posterior probabilities (PP) > 0.84 are shown at the nodes and thickened lines represent nodes with PP = 1.00. The scale bar represents the expected changes per site. Families and orders are indicated with coloured blocks to the right of the tree. GenBank accession and/or Fungal Planet numbers are indicated behind the species names. The tree was rooted to Saccharata proteae (GenBank EU552145.1) and the taxonomic novelties described in this study for which LSU sequence data were available are indicated in bold face. The alignment and tree were deposited in TreeBASE (Submission ID S25229).
Overview Xylariales (Sordariomycetes) phylogeny – part 1.
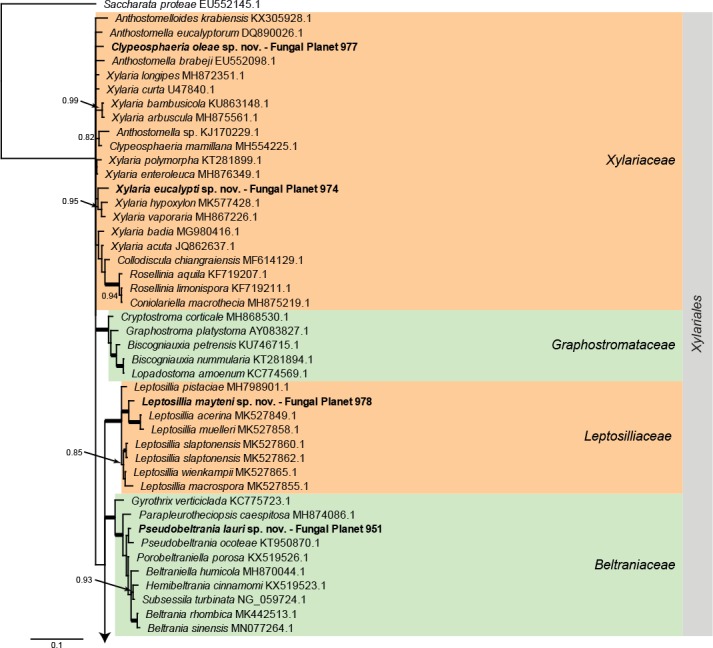
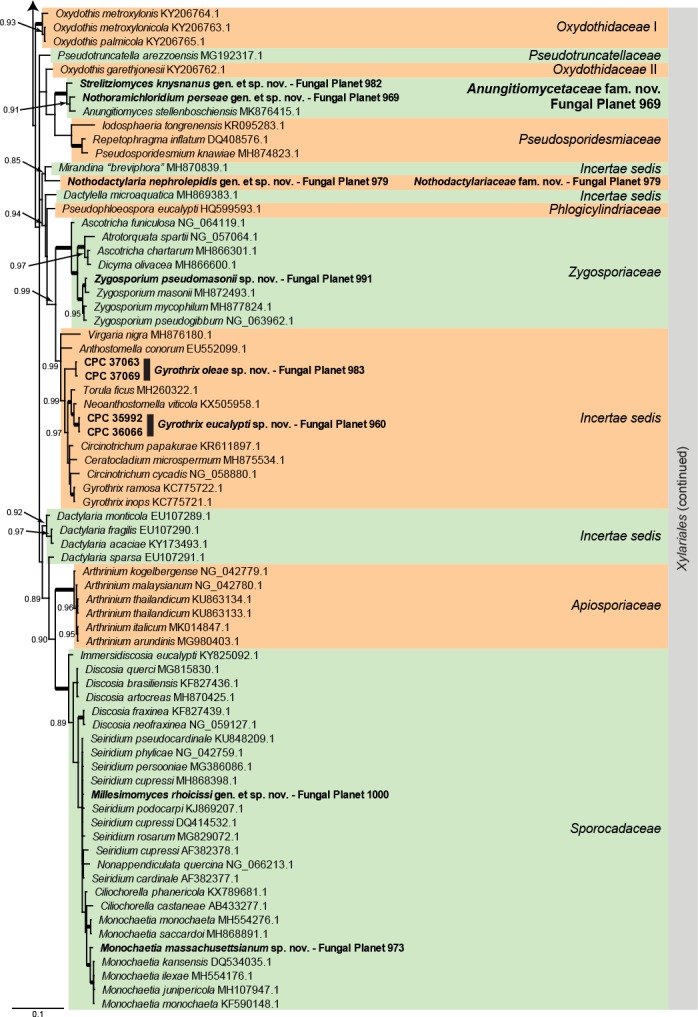
Consensus phylogram (50 % majority rule) of 25 278 trees resulting from a Bayesian analysis of the LSU sequence alignment (117 sequences including outgroup; 899 aligned positions; 248 unique site patterns) using MrBayes v. 3.2.6 (Ronquist et al. 2012). Bayesian posterior probabilities (PP) > 0.84 are shown at the nodes and thickened lines represent nodes with PP = 1.00. The scale bar represents the expected changes per site. Families and orders are indicated with coloured blocks to the right of the tree. GenBank accession and/or Fungal Planet numbers are indicated behind the species names. The tree was rooted to Saccharata proteae (GenBank EU552145.1) and the taxonomic novelties described in this study for which LSU sequence data were available are indicated in bold face. The alignment and tree were deposited in TreeBASE (Submission ID S25229).
Acknowledgements
Gabriel Moreno and colleagues express their gratitude to Dr L. Monje and A. Pueblas of the Department of Drawing and Scientific Photography at the University of Alcalá for their help in the digital preparation of the photographs. Dr J. Rejos, curator of the AH herbarium, assisted with the specimens examined in the present study, and members of the Sociedad Micológica de Madrid provided many specimens included in this study, as well as Patrick Chapon, André Dudoret, Véronique Dumas, Geneviève Essertier, Jean-Luc Fasciotto, Jean-Marc Moingeon and Franck Richard. Some of the DNA sequencing was performed by the Mycoseq partnership between the Société Mycologique de France and the Centre d’Ecologie Fonctionnelle et Evolutive of Montpellier. The study of E. Malysheva, V. Malysheva, O.V. Morozova and E.S. Popov was conducted as part of a research project (no. AAAA-A19-119020890079-6) of the Komarov Botanical Institute of the Russian Academy of Sciences using equipment of its Core Facility Centre ‘Cell and Molecular Technologies in Plant Science’. The study of Alina V. Alexandrova was carried out with the support of the RUDN University Program 5-100. Financial support was provided to Ana C.M. Rodrigues by the Coordination of Improvement of Higher Level Personnel (CAPES); thanks to the National Council for Scientific and Technological Development (CNPq) for CNPq-Universal 2016 grant (409960/2016-0) and for CNPq-Special Visiting Researcher grant (407474/2013-7). The study of Desirrê A.L. Petters-Vandresen and colleagues was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) - Finance Code 001. CAPES also provided a master scholarship for D.A.L. Petters-Vandresen at the Programa de Pós-Graduação em Genética (Universidade Federal do Paraná – UFPR). Thanks are also given to the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for providing a research fellowship to Chirlei Glienke and Lygia V.G. Terasawa. They also thank Vértika Agropecuária for providing the maize samples, as well as technical and scientific support during the research. Daniel P. Bruschi, Daiani C. Savi, Lucimeris Ruaro and Rodrigo Aluizio are also thanked for suggestions and assistance. Ruane V.B. Araújo and colleagues thank the Laboratory of Electron Microscopy of the Universidade Federal da Bahia (LAMUME/UFBA) for their collaboration with Scanning Electron Microscopy. Financial support was provided by the Universidade Federal da Bahia. Saúl De la Peña-Lastra and Pablo Alvarado thank the Atlantic Islands National Maritime-Terrestrial Park authorities and guards, especially Víctor R. Iglesias. They also thank Enrique Rubio and Hans-Otto Baral for their help with the identification, Enrique Rubio for the microscopy, and Óscar Requejo for the assembly of photos. Carlos Gil-Durán received a doctoral fellowship, CONICYT-PFCHA/Doctorado Nacional/2014-63140056. Inmaculada Vaca acknowledges the support of project INACH RG_15-14. Renato Chávez acknowledges the support of DICYT-USACH and project INACH RG_03-14. Michael Loizides and colleagues thank Franck Richard for the habitat photo at Pézilla-de-Conflent, Chenil Sauvage, and Dr Konstanze Bensch and Dr Shaun Pennycook for advice on nomenclatural issues. Jennifer Luangsa-ard and colleagues thank Prof. Morakot Tanticharoen, Dr Somvong Tragoonrung, and the Platform Technology Management Section, National Centre for Genetic Engineering and Biotechnology (BIOTEC), Grant No. P-19-50231 and CPMO Grant No. P15-51452 for their support of the program Biodiversity studies of entomopathogenic fungi in Thailand. They also thank Artit Khonsanit for photos of the natural samples and Sarunyou Wongkanoun for photos of the type locality. The study of Daniel Torres-Garcia and colleagues was partially supported by the Spanish Ministerio de Economía, Industria y Competitividad (grant CGL2017-88094-P). Lynn Delgat was funded by a doctoral scholarship of the Special Research Fund (grant BOFDOC2015007001). Jean Lodge’s research was supported by a National Science Foundation Biotic Surveys and Inventories Grant (DEB-9525902) to the Research Foundation of SUNY Cortland in a joint venture agreement with the USDA Forest Service Forest Products Laboratory, and the Conservation Agency and Falconwood Foundation for food, housing and local transportation on Guana Island. Mélanie Roy is thanked for sequencing, which was funded by the Laboratoire d’Excellence CEBA (ANR-10-LABX-25-01). The research of Cobus M. Visagie was supported by a grant from the NRF-FBIP Program (grant nr FBIS170605237212). Abigail E. Rea and colleagues acknowledge the National Fish & Wildlife Foundation, the Pennsylvania Game Commission, Lock Haven University, Temple University, as well as Dr. Joseph Calabrese, Natasha Ortiz, Jacob Adam, Kayla Riehle and Eric Shuffelbottom. Antonio Rodríguez and colleagues are grateful to AEI/FEDER, UE (CGL2016-78946-R) and Fundación Séneca-Agencia de Ciencia y Tecnología de la Región de Murcia (20866/PI/18) for financial support. Juan Julián Bordallo thanks Asunción Morte (Universidad de Murcia, Spain) for assistance (projects CGL2016-78946-R and 20866/PI/18). The study of Isabel Iturrieta-González and colleagues was partially supported by the Spanish Ministerio de Economía, Industria y Competitividad (grant CGL2017-88094-P). Nataliya E. Ivanushkina and colleagues acknowledge Elizaveta Rivkina for the background photograph. Anastasiya Danilogorskaya and Irina Pinchuk are thanked for assisting with the molecular analyses, and the Russian Foundation for Basic Research (grant 18-04-01347-a) is acknowledged for funding. Fang Liu acknowledges the National Natural Science Foundation of China (NSFC31770009) for financial support. The research of Slavomír Adamčík, Miroslav Caboň and Munazza Kiran was funded by the National project APVV 15-0210; the study was prepared during the fellowship of Munazza Kiran at the Plant Science and Biodiversity Centre of the Slovak Academy of Sciences awarded by the National Scholarship Program of the Slovak Republic.
Supplementary material
References
- Al-Hatmi AMS, Bonifaz A, De Hoog GS, et al. 2014. Keratitis by Fusarium temperatum, a novel opportunist. BMC Infectious Diseases 14: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andjic V, Carnegie AJ, Pegg GS, et al. 2019. 23 years of research on Terato-sphaeria leaf blight of Eucalyptus. Forest Ecology and Management 443: 19–27. [Google Scholar]
- Aveskamp MM, Guyter J, Woudenberg JHC, et al. 2010. Highlights of the Didymellaceae: A polyphasic approach to characterise Phoma and related pleosporalean genera. Studies in Mycology 65: 1–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baral H-O-, Rämä T. 2015. Morphological update on Calycina marina (Pezizellaceae, Helotiales, Leotiomycetes), a new combination for Laetinaevia marina. Botanica Marina 58 (6): 523–534. [Google Scholar]
- Barbosa RN, Bezerra JDP, Souza-Motta CM, et al. 2018. New Penicillium and Talaromyces species from honey, pollen and nests of stingless bees. Antonie van Leeuwenhoek 111: 1883–1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batista AC, Bezerra JL, Da Silva MH. 1960. Vonarxia n.gen.e outros imperfecti fungi. Publica[ccedil]ões Instituto de Micologia da Universidade do Recife 283: 1–32. [Google Scholar]
- Bensch K, Braun U, Groenewald JZ, et al. 2012. The genus Cladosporium. Studies in Mycology 72: 1–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensch K, Groenewald JZ, Braun U, et al. 2015. Common but different: The expanding realm of Cladosporium. Studies in Mycology 82: 23–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessette A, Roody WC, Bessette AR. 1999. North American Boletes: a color guide to the fleshy pored mushrooms. Syracuse University Press. [Google Scholar]
- Boertmann D. 1990. The identity of Hygrocybe vitellina and related species. Nordic Journal of Botany 10: 311–317. [Google Scholar]
- Boertmann D. 2010. The genus Hygrocybe. Fungi of Northern Europe vol. I, 2nd edn Danish Mycological Society, Denmark. [Google Scholar]
- Bordallo JJ, Rodríguez A, Kaounas V, et al. 2015. Two new Terfezia species from Southern Europe. Phytotaxa 230: 239–249. [Google Scholar]
- Bordallo JJ, Rodríguez A, Muñoz-Mohedano JM, et al. 2013. Five new Terfezia species from the Iberian Peninsula. Mycotaxon 124: 189–208. [Google Scholar]
- Borgen T, Arnolds E. 2004. Taxonomy, ecology and distribution of Hygrocybe (Fr.) P. Kumm and Camarophyllopsis Herink (Fungi, Basidiomycota, Hygrocybeae) in Greenland. Bioscience 54: 1–68. [Google Scholar]
- Bourdot H, Galzin A. 1928. Hyménomycètes de France. Bry M. ed., Sceaux, F. [Google Scholar]
- Braun U, Cook RTA. 2012. Taxonomic manual of the Erysiphales (Powdery Mildews). CBS Biodiversity Series 11. CBS, Utrecht, Netherlands. [Google Scholar]
- Braun U, Shin HD, Takamatsu S, et al. 2019. Phylogeny and taxonomy of Golovinomyces orontii revisited. Mycological Progress 18: 335–357. [Google Scholar]
- Bulliard JBP. 1791. Histoire des champignons de la France I: 1–368. [Google Scholar]
- Caboň M, Li GJ, Saba M, et al. 2019. Phylogenetic study documents different speciation mechanisms within the Russula globispora lineage in boreal and arctic environments of the northern hemisphere. IMA Fungus 1: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabral TS, Silva BDB, Ishkawa NK, et al. 2014. A new species and new records of gasteroid fungi (Basidiomycota) from Central Amazonia, Brazil. Phytotaxa 183: 239–253. [Google Scholar]
- Cai L, Wu W-P-, Hyde KD. 2009. Phylogenetic relationships of Chalara and allied species inferred from ribosomal DNA sequences. Mycological Progress 8: 133–143. [Google Scholar]
- Calonge FD, Demoulin V. 1975. Les Gastéromycètes d’Espagne. Bulletin Trimestriel de la Société Mycologique de France 91: 247–292. [Google Scholar]
- Calonge FD, Mata M. 2004. A new species of Geastrum from Costa Rica and Mexico. Boletín de la Sociedad Micológica de Madrid 28: 331–335. [Google Scholar]
- Candusso M. 1997. Hygrophorus s. l. Fungi Europaei 6: 1–784. [Google Scholar]
- Cheewangkoon R, Groenewald JZ, Summerell BA, et al. 2009. Myrtaceae, a cache of fungal biodiversity. Persoonia 23: 55–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomnunti P, Ko TWK, Chukeatirote E, et al. 2012. Phylogeny of Chaetothyriaceae in northern Thailand including three new species. Mycologia 104: 382–395. [DOI] [PubMed] [Google Scholar]
- Consiglio G, Contu M. 2007. Hygrocybe citrinopallida. Rivista di Micologia 1: 57–64. [Google Scholar]
- Cooper J, Leonard P. 2012. Boletopsis nothofagi sp. nov. associated with Nothofagus in the Southern Hemisphere. MycoKeys 3: 13–22. [Google Scholar]
- Corner EJH. 1950. A monograph of Clavaria and allied genera. Oxford University Press, London, UK. [Google Scholar]
- Corner EJH. 1970. Supplement to ‘A monograph of Clavaria and allied genera’. Beihefte zur Nova Hedwigia 33: 1–299. [Google Scholar]
- Corner EJH. 1972. Boletus in Malaysia. Botanic Gardens, Singapore. [Google Scholar]
- Costa MLN, Machado JC, Guimarães RM, et al. 2003. Inoculação de Fusarium oxysporum f. sp. phaseoli em sementes de feijoeiro através de restrição hídrica. Ciência e Agrotecnologia 27: 1023–1030. [Google Scholar]
- Crous PW, Braun U. 2003. Mycosphaerella and its anamorphs. 1. Names published in Cercospora and Passalora. CBS Biodiversity Series 1: 1–571. CBS-KNAW Fungal Biodiversity Centre, Utrecht, Netherlands. [Google Scholar]
- Crous PW, Braun U, Groenewald JZ. 2007a. Mycosphaerella is polyphyletic. Studies in Mycology 58: 1–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Braun U, Hunter GC, et al. 2013a. Phylogenetic lineages in Pseudocercospora. Studies in Mycology 75: 37–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Braun U, Schubert K, et al. 2007b. Delimiting Cladosporium from morphologically similar genera. Studies in Mycology 58: 33–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Carnegie AJ, Wingfield MJ, et al. 2019a. Fungal Planet description sheets: 868–950. Persoonia 42: 291–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Kendrick WB, Alfenas AC. 1997. New species of hyphomycetes associated with Eucalyptus. South African Journal of Botany 63: 286–290. [Google Scholar]
- Crous PW, Luangsa-ard JJ, Wingfield MJ, et al. 2018a. Fungal Planet description sheets: 785–867. Persoonia 41: 238–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Schubert K, Braun U, et al. 2007c. Opportunistic, human-pathogenic species in the Herpotrichiellaceae are phenotypically similar to saprobic or Phytopathogenic species in the Venturiaceae. Studies in Mycology 58: 185–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Schumacher RK, Akulov A, et al. 2019b. New and interesting fungi. 2. Fungal Systematics and Evolution 3: 57–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Schumacher RK, Wingfield MJ, et al. 2015a. Fungal Systematics and Evolution: FUSE 1. Sydowia 67: 81–118. [Google Scholar]
- Crous PW, Schumacher RK, Wingfield MJ, et al. 2018b. New and interesting fungi. 1. Fungal Systematics and Evolution 1: 169–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Shivas RG, Quaedvlieg W, et al. 2014. Fungal Planet description sheets: 214–280. Persoonia 32: 184–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Shivas RG, Wingfield MJ, et al. 2012a. Fungal Planet description sheets: 128–153. Persoonia 29: 146–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Summerell BA, Shivas RG, et al. 2012b. Fungal Planet description sheets: 107–127. Persoonia 28: 138–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Wingfield MJ. 1993. A re-evaluation of Cylindrocladiella, and a comparison with allied genera. Mycological Research 97: 433–448. [Google Scholar]
- Crous PW, Wingfield MJ, Alfenas AC, et al. 1994. Cylindrocladium naviculatum sp. nov., and two new vesiculate Hyphomycete genera, Falcocladium and Vesicladiella. Mycotaxon 50: 441–458. [Google Scholar]
- Crous PW, Wingfield MJ, Burgess TI, et al. 2016a. Fungal Planet description sheets: 232–233. Persoonia 37: 469–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Wingfield MJ, Burgess TI, et al. 2017a. Fungal Planet description sheets: 625–715. Persoonia 39: 270–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Wingfield MJ, Burgess TI, et al. 2017b. Fungal Planet description sheets: 558–624. Persoonia 38: 240–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Wingfield MJ, Burgess TI, et al. 2018c. Fungal Planet description sheets: 716–784. Persoonia 40: 240–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Wingfield MJ, Cheewangkoon R, et al. 2019c. Foliar pathogens of eucalypts. Studies in Mycology 94: 125–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Wingfield MJ, Guarro J, et al. 2013b. Fungal Planet description sheets: 154–213. Persoonia 31: 188–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Wingfield MJ, Guarro J, et al. 2015b. Fungal Planet description sheets: 320–370. Persoonia 34: 167–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Wingfield MJ, Richardson DM, et al. 2016b. Fungal Planet description sheets: 400–468. Persoonia 36: 316–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damm U, Cannon PF, Woudenberg JHC, et al. 2012. The Colletotrichum acutatum species complex. Studies in Mycology 73: 37–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Crop E, Nuytinck J, Van de Putte K, et al. 2017. A multi-gene phylogeny of Lactifluus (Basidiomycota, Russulales) translated into a new infrageneric classification of the genus. Persoonia 38: 58–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Hoog GS. 1985. Taxonomy of the Dactylaria complex, IV. Dactylaria, Neta, Subulispora and Scolecobasidium. Studies in Mycology 26: 1–60. [Google Scholar]
- Decock C, Delgado-Rodríguez G, Buchet S, et al. 2003. A new species and three new combinations in Cyphellophora, with a note on the taxonomic affinities of the genus, and its relation to Kumbhamaya and Pseudomicrodochium. Antonie van Leeuwenhoek 84: 209–216. [DOI] [PubMed] [Google Scholar]
- Demoulin V. 1979. The typification of Lycoperdon described by Peck and Morgan. Beihefte zur Sydowia 8: 139–151. [Google Scholar]
- Dereeper A, Guignon V, Blanc G, et al. 2008. Phylogeny.fr: robust phylogenetic analysis for the non-specialist. Nucleic Acids Research (Web Server issue): W465-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desprez-Loustau M-L, Massot M, Toïgo M, et al. 2018. From leaf to continent: the multi-scale distribution of an invasive cryptic pathogen complex on oak. Fungal Ecology 36: 39–50. [Google Scholar]
- Domsch K, Gams W, Anderson TH. 2007. Compendium of soil fungi, 2 edn IHW-Verlag, Eching. [Google Scholar]
- Donk MA. 1933. Revisie van de Nederlandse Heterobasidiomyceteae (uitgez. Uredinales en Ustilaginales) en Homobasidiomyceteae-Aphyllophoraceae: II. Mededelingen van het botanisch Museum en Herbarium van de Rijksuniversiteit Utrecht 9: 1–278. [Google Scholar]
- Dugan FM, Roberts RG, Hanlin RT. 1995. New and rare fungi from cherry fruits. Mycologia 87: 713–718. [Google Scholar]
- Ellis MB. 1971. Dematiaceous Hyphomycetes. Commonwealth Mycological Institute, Kew, Surrey, England. [Google Scholar]
- Ellis MB. 1976. More Dematiaceous Hyphomycetes. Commonwealth Mycological Institute, Kew, Surrey, England. [Google Scholar]
- Ellison CA, Sankaran KV. 2017. Profile of an invasive plant: Mikania micrantha. In: Ellison CA, Sankaran KV, Murphy ST. (eds), Invasive alien plants: 18–28. CABI Publishing, Wallingford, UK. [Google Scholar]
- Eriksson OE. 1982. Cordyceps bifusispora spec. nov. Mycotaxon 15: 185–188. [Google Scholar]
- Faria CB, Abe CAL, Da Silva CN, et al. 2012. New PCR assays for the identification of Fusarium verticillioides, Fusarium subglutinans, and other species of the Gibberella fujikuroi complex. International Journal of Molecular Sciences 13: 115–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funnell-Harris DL, Scully ED, Sattler SE, et al. 2017. Differences in Fusarium species in brown midrib Sorghum and in air populations in production fields. Phytopathology 107: 1353–1363. [DOI] [PubMed] [Google Scholar]
- Giraldo A, Crous PW. 2019. Inside Plectosphaerellaceae. Studies in Mycology 92: 227–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraldo A, Hernández-Restrepo M, Crous PW. 2019. New plectosphaerellaceous species from Dutch garden soil. Mycological Progress 18: 1135–1154. [Google Scholar]
- Gómez LD, Singer R. 1984. Veloporphyrellus, a new genus of Boletaceae from Costa Rica. Brenesia 22: 293–298. [Google Scholar]
- Gouy M, Guindon S, Gascuel O. 2010. SeaView version 4: A multiplatform graphical user interface for sequence alignment and phylogenetic tree building. Molecular Biology and Evolution 27: 221–224. [DOI] [PubMed] [Google Scholar]
- Guatimosim E, Schwartsburd PB, Barreto RW, et al. 2016. Novel fungi from an old niche: cercosporoid and related sexual morphs on ferns. Persoonia 37: 106–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guindon S, Gascuel O. 2003. A simple, fast and accurate algorithm to estimate large phylogenies by maximum likelihood. Systematic Biology 52: 696–704. [DOI] [PubMed] [Google Scholar]
- Han J-G-, Hosoya T, Sung G-H, et al. 2014. Phylogenetic reassessment of Hyaloscyphaceae sensu lato (Helotiales, Leotiomycetes) based on multigene analyses. Fungal Biology 118: 150–167. [DOI] [PubMed] [Google Scholar]
- Hausknecht A, Krisai-Greilhuber I, Jaklitsch W. 2003. Rezente Pilfunde aus Osttirol. Österreischiche Zeitschrifte für Pilzkunde 12: 153–192. [Google Scholar]
- Hernández-Restrepo M, Gené J, Castañeda-Ruiz RF, et al. 2017. Phylogeny of saprobic microfungi from Southern Europe. Studies in Mycology 86: 53–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heykoop M, Moreno G, Alvarado P, et al. 2017. El género Psathyrella (Fr.) Quél. S.l. en España. VI. Especies nuevas o raras y reevaluación de otras. Boletín de la Sociedad Micológica de Madrid 41: 71–98. [Google Scholar]
- Holubová-Jechová V. 1974. A revision of the genus Olpitrichum Atk. Folia Geobotanica et Phytotaxonomica 9: 425–432. [Google Scholar]
- Hughes SJ. 1958. Revisiones hyphomycetum aliquot cum appendice de nominibus rejiciendis. Canadian Journal of Botany 36: 727–836. [Google Scholar]
- Hughes SJ, Pirozynski KA. 1971. New Zealand fungi 15. Beltraniella, Circinotrichum, and Gyrothrix (Syn. Peglionia). New Zealand Journal of Botany 9: 39–45. [Google Scholar]
- Hustad VP, Miller AN. 2011. Phylogenetic placement of four genera within the Leotiomycetes (Ascomycota). North American Fungi 6: 1–13. [Google Scholar]
- Isola D, Zucconi L, Onofri S, et al. 2016. Extremotolerant rock inhabiting black fungi from Italian monumental sites. Fungal Diversity 76: 75–96. [Google Scholar]
- Jaklitsch WM, Checa J, Blanco MN, et al. 2018. A preliminary account of the Cucurbitariaceae. Studies in Mycology 90: 71–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaklitsch WM, Gardiennet A, Voglmayr H. 2016. Resolution of morphology-based taxonomic delusions: Acrocordiella, Basiseptospora, Blogiascospora, Clypeosphaeria, Hymenopleella, Lepteutypa, Pseudapiospora, Requienella, Seiridium and Strickeria. Persoonia 37: 82–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaklitsch WM, Samuels GL, Dodd SL, et al. 2006. Hypocrea rufa/Trichoderma viride: a reassessment, and description of five closely related species with and without warted conidia. Studies in Mycology 56: 135–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang JR, Cai L, Liu F. 2017. Oligotrophic fungi from a carbonate cave, with three new species of Cephalotrichum. Mycology 8: 164–177. [Google Scholar]
- Jiang Y, Dukik K, Muñoz JF, et al. 2018. Phylogeny, ecology and taxonomy of systemic pathogens and their relatives in Ajellomycetaceae (Onygenales): Blastomyces, Emergomyces, Emmonsia, Emmonsiellopsis. Fungal Diversity 90: 245–291. [Google Scholar]
- Johnston PR, Quijada L, Smith CA, et al. 2019. A multigene phylogeny toward a new phylogenetic classification of Leotiomycetes. IMA Fungus 10: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karsten PA. 1871. Mycologia fennica. Pars prima. Discomycetes. Bidrag till Kännedom av Finlands Natur och Folk 19: 1–264. [Google Scholar]
- Karsten PA. 1873. Mycologia fennica. Pars secunda. Pyrenomycetes. Bidrag till Kännedom av Finlands Natur och Folk 23: 1–252. [Google Scholar]
- Kim JH, Kang MR, Kim HK, et al. 2012. Population structure of the Gibberella fujikuroi species complex associated with rice and corn in Korea. Plant Pathology Journal 28: 357–363. [Google Scholar]
- Kirschstein W. 1938. Über neue, seltene und kritische Ascomyceten und Fungi imperfecti. I. Annales Mycologici 36: 367–400. [Google Scholar]
- Kiss L, Jankovics T, Kovács GM, et al. 2008. Oidium longipes, a new powdery mildew fungus on petunia in the USA: A potential threat to ornamental and vegetable solanaceous crops. Plant Disease 92: 818–825. [DOI] [PubMed] [Google Scholar]
- Kits van Waveren E. 1985. The Dutch, French and British species of Psathyrella. Persoonia Supplement 2: 1–300. [Google Scholar]
- Kornerup A, Wanscher JH. 1967. Methuen Handbook of Colour. Methuen & Co Ltd, London. [Google Scholar]
- Kornerup A, Wanscher JH. 1978. Methuen Handbook of Colour. 3rd ed Eyre Methuen, London. [Google Scholar]
- Kreisel H. 1967. Taxonomisch-Pflanzengeographische Monographie der Gattung Bovista. Beiheft Nova Hedwigia 25: 1–244. [Google Scholar]
- Kumar S, Stecher G, Li M, et al. 2018. MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Molecular Biology and Evolution 35: 1547–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Küppers H. 2002. Atlas de los colores. Blume: 1–165. [Google Scholar]
- Larsson E, Örstadius L. 2008. Fourteen coprophilous species of Psathyrella identified in the Nordic countries using morphology and nuclear rDNA sequence data. Mycological Research 112: 1165–1185. [DOI] [PubMed] [Google Scholar]
- Le Gal F, Mangenot F. 1958. Revue mycologique (Paris) 23: 54. [Google Scholar]
- Leslie JF, Summerell BA. 2006. The Fusarium Laboratory Manual. Blackwell Publishing, Ames, Iowa, USA. [Google Scholar]
- Li GJ, Zhang CL, Zhao RL, et al. 2018. Two new species of Russula from Northeast China. Mycosphere 9: 431–443. [Google Scholar]
- Li YC, Ortiz-Santana B, Zeng NK, et al. 2014. Molecular phylogeny and taxonomy of the genus Veloporphyrellus. Mycologia 106: 291–306. [DOI] [PubMed] [Google Scholar]
- Liu F, Bonthond G, Groenewald JZ, et al. 2019a. Sporocadaceae, a family of coelomycetous fungi with appendage-bearing conidia. Studies in Mycology 92: 287–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Wang J, Li H, et al. 2019b. Setophoma spp. on Camellia sinensis. Fungal Systematics and Evolution 4: 43–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge D, Padamsse M, Matheny PB, et al. 2013. Molecular phylogeny, morphology, pigment chemistry and ecology in Hygrophoraceae (Agaricales). Fungal Diversity 10: 311–317. [Google Scholar]
- Lombard L, Shivas RG, To-Anun C, et al. 2012. Phylogeny and taxonomy of the genus Cylindrocladiella. Mycological Progress 11: 835–868. [Google Scholar]
- Luangsa-ard JJ, Tasanathai K, Mongkolsamrit S, et al. 2008. Atlas of Invertebrate-Pathogenic Fungi of Thailand, vol. 2. NSTDA publication. Darnsutha Press Co., Ltd. [Google Scholar]
- Malysheva EF, Malysheva VF, Justo A. 2016. Observation on Pluteus (Pluteaceae) diversity in South Siberia, Russia: morphological and molecular data. Mycological Progress 15: 861–882. [Google Scholar]
- Marin-Felix Y, Groenewald JZ, Cai L, et al. 2017. Genera of phytopathogenic fungi: GOPHY 1. Studies in Mycology 86: 99–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin-Felix Y, Hernández-Restrepo M, Iturrieta-González I, et al. 2019. Genera of phytopathogenic fungi: GOPHY 3. Studies in Mycology 94: 1–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin-Felix Y, Stchigel AM, Cano-Lira JF, et al. 2015. Emmonsiellopsis, a new genus related to the thermally dimorphic fungi of the family Ajellomycetaceae. Mycoses 58: 451–460. [DOI] [PubMed] [Google Scholar]
- Menolli N, Jr, Justo A, Capelari M. 2015. Phylogeny of Pluteus section Celluloderma including eight new species from Brazil. Mycologia 107: 1205–1220. [DOI] [PubMed] [Google Scholar]
- Miller AN, Huhndorf SM. 2004. A natural classification of Lasiosphaeria based on nuclear LSU rDNA sequences. Mycological Research 108: 26–34. [DOI] [PubMed] [Google Scholar]
- Miller AN, Huhndorf SM, Fournier J. 2014. Phylogenetic relationships of five uncommon species of Lasiosphaeria and three new species in the Helminthosphaeriaceae (Sordariomycetes). Mycologia 106: 505–524. [DOI] [PubMed] [Google Scholar]
- Miller MA, Pfeiffer W, Schwartz T. 2010. Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In: Proceedings of the Gateway Computing Environments Workshop (GCE), New Orleans, LA: 1–8. [Google Scholar]
- Miller MA, Pfeiffer W, Schwartz T. 2012. The CIPRES science gateway: enabling high-impact science for phylogenetics researchers with limited resources. In: Proceedings of the 1st Conference of the Extreme Science and Engineering Discovery Environment: Bridging from the extreme to the campus and beyond: 1–8. Association for Computing Machinery, USA. [Google Scholar]
- Miller OK, Lodge DJ, Baroni TJ. 2000. New and interesting ectomycorrhizal fungi from Puerto Rico, Mona, and Guana Islands. Mycologia 92: 558–570. [Google Scholar]
- Minnis AM, Lindner DL. 2013. Phylogenetic evaluation of Geomyces and allies reveals no close relatives of Pseudogymnoascus destructans, comb. nov., in bat hibernacula of eastern North America. Fungal Mycology 117: 638–649. [DOI] [PubMed] [Google Scholar]
- Mongkolsamrit S, Noisripoom W, Thanakitpipattana D, et al. 2018. Disentangling cryptic species with isaria-like morphs in Cordycipitaceae. Mycologia 110: 230–257. [DOI] [PubMed] [Google Scholar]
- Moyersoen B, Demoulin V. 1996. Les Gastéromycètes de Corse: taxonomie, écologie, chorologie. Lejeunia 152: 1–130. [Google Scholar]
- Müller W, Agerer R. 1990. Studien an Ektomykorrhizen. XXIX, Drei Mykorrhizen aus der Leccinum-scabrum-Gruppe. Nova Hedwigia 51: 381–410. [Google Scholar]
- Nag Raj TR. 1993. Coelomycetous anamorphs with appendage-bearing conidia. Mycologue Publications, Waterloo, Ontario. [Google Scholar]
- Nag Raj TR, Kendrick B. 1976. A monograph of Chalara and allied genera. Wilfrid Laurier University Press, Waterloo, Ontario, Canada. [Google Scholar]
- Nagy LG, Vágvölgyi C, Papp T. 2013. Morphological characterization of clades of the Psathyrellaceae (Agaricales) inferred from a multigene phylogeny. Mycological Progress 12: 505–517. [Google Scholar]
- Nagy LG, Walther G, Házi J, et al. 2011. Understanding the evolutionary processes of fungal fruiting bodies: correlated evolution and divergence times in the Psathyrellaceae. Systematic Biology 60: 303–317. [DOI] [PubMed] [Google Scholar]
- Nguyen L-T, Schmidt HA, Von Haeseler A, et al. 2015. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum likelihood phylogenies. Molecular Biology and Evolution 32: 268–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemelä T, Saarenoksa R. 1989. On Fennoscandian polypores 10. Boletopsis leucomelaena and B. grisea described and illustrated. Karstenia 29: 12–28. [Google Scholar]
- Nuangmek W, Aiduang W, Suwannarach N, et al. 2018. First report of gummy stem blight caused by Stagonosporopsis cucurbitacearum on cantaloupe in Thailand. Canadian Journal of Plant Pathology 40: 306–311. [Google Scholar]
- O’Donnell K, McCormick SP, Busman M, et al. 2018. Marasas et al. 1984. ‘Toxigenic Fusarium Species: Identity and Mycotoxicology’ revisited. Mycologia 110: 1058–1080. [DOI] [PubMed] [Google Scholar]
- Olariaga I, Jugo BM, Garcia-Etxerbarria K, et al. 2009. Species delimitation in the European species of Clavulina (Cantharellales, Basidiomycota) inferred from phylogenetic analyses of ITS region and morphological data. Mycological Research 113: 1261–1270. [DOI] [PubMed] [Google Scholar]
- Olariaga I, Salcedo I. 2012. New combinations and notes in clavarioid fungi. Mycotaxon 121: 37–44. [Google Scholar]
- Olson DM, Dinerstein E, Wikramanayake ED, et al. 2001. Terrestrial ecoregions of the world: A new map of life on earth. BioScience 51: 933–938. [Google Scholar]
- Örstadius L, Ryberg M, Larsson E. 2015. Molecular phylogenetics and taxonomy in Psathyrellaceae (Agaricales) with focus on psathyrelloid species: introduction of three new genera and 18 new species. Mycological Progress 14: 25. [Google Scholar]
- Peterson SW, Vega F, Posada F, et al. 2005. Penicillium coffeae, a new endophytic species isolated from a coffee plant and its phylogenetic relationship to P. fellutanum, P. thiersii and P. brocae based on parsimony analysis of multilocus DNA sequences. Mycologia 97: 659–666. [DOI] [PubMed] [Google Scholar]
- Petrak F, Sydow H. 1934. Kritisch-systematische Originaluntersuchungen über Pyrenomyzeten, Sphaeropsideen und Melanconieen. Annales Mycologici 32: 1–35. [Google Scholar]
- Pham NQ, Barnes I, Chen SF, et al. 2018. New species of Cylindrocladiella from plantation soils in South-East Asia. MycoKeys 32: 1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitt JI. 1980. The genus Penicillium and its teleomorphic states Eupenicillium and Talaromyces. Academic Press, London. [Google Scholar]
- Pratibha SJ, Gawas P, Shenoy BD, et al. 2005. Chalara indica sp. nov. and Sorocybe indicus sp. nov. from India. Cryptogamie, Mycologie 26: 97–103. [Google Scholar]
- Quaedvlieg W, Binder M, Groenewald JZ, et al. 2014. Introducing the consolidated species concept to resolve species in the Teratosphaeriaceae. Persoonia 33: 1–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaedvlieg W, Verkley GJM, Shin H-D-, et al. 2013. Sizing up Septoria. Studies in Mycology 75: 307–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajeshkumar KC, Crous PW, Groenewald JZ, et al. 2016. Resolving the phylogenetic placement of Porobeltraniella and allied genera in the Beltraniaceae. Mycological Progress 15: 1119–1136. [Google Scholar]
- Rayner RW. 1970. A mycological colour chart. Commonwealth Mycological Institute & British Mycological Society, Kew, Richmond. [Google Scholar]
- Rea C. 1918. New or rare British fungi. Transactions of the British Mycological Society 6: 61–64. [Google Scholar]
- Richter T, Baral H-O. 2008. Coronellaria pulicaris, Mollisia luctuosa und Marasmius corelii - seltene Saprobionten an Cyperaceen. Boletus 31: 45–63. [Google Scholar]
- Ronquist F, Huelsenbeck JP. 2003. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19: 1572–1574. [DOI] [PubMed] [Google Scholar]
- Ronquist F, Teslenko M, Van der Mark P, et al. 2012. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology 61: 539–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossman AY, Aime MC, Farr DF, et al. 2004. The coelomycetous genera Chaetomella and Pilidium represent a newly discovered lineage of inoperculate discomycetes. Mycological Progress 3: 275–290. [Google Scholar]
- Rossman AY, Crous PW, Hyde KD, et al. 2015. Recommended names for pleomorphic genera in Dothideomycetes. IMA Fungus 6: 507–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samerpitak K, Van der Linde E, Choi H-J, et al. 2014. Taxonomy of Ochroconis, genus including opportunistic pathogens on humans and animals. Fungal Diversity 65: 89–126. [Google Scholar]
- Samson RA. 1972. Notes on Pseudogymnoascus, Gymnoascus and related genera. Acta Botanica Neerlandica 21: 517–527. [Google Scholar]
- Samuels GJ, Dodd SL, Lu B-S-, et al. 2006. The Trichoderma koningii aggregate species. Studies in Mycology 56: 67–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez L, Gibert YS. 2015. Chromosera viola (J. Geesink & Bas) Vizzini & Ercole 2012, une spectacular especie localizada en Cataluña. Revista Catalana de Micologia 36: 29–32. [Google Scholar]
- Scauflaire J, Gourgue M, Munaut F. 2011. Fusarium temperatum sp. nov. from maize, an emergent species closely related to Fusarium subglutinans. Mycologia 103: 586–597. [DOI] [PubMed] [Google Scholar]
- Scholler M, Schmidt A, Siahaan SAS, et al. 2016. A taxonomic and phylogenetic study of the Golovinomyces biocellatus complex (Erysiphales, Ascomycota) using asexual state morphology and rDNA sequence data. Mycological Progress 15: 56. [Google Scholar]
- Schubert K, Groenewald Z, Braun U, et al. 2007. Biodiversity in the Cladosporium herbarum complex (Davidiellaceae, Capnodiales), with standardization of methods for Cladosporium taxonomy and diagnostics. Studies in Mycology 58: 105–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultes NP, Murtishi B, Li DW. 2017. Phylogenetic relationships of Chlamydomyces, Harzia, Olpitrichum, and their sexual allies, Melanospora and Sphaerodes. Fungal Biology 121: 890–904. [DOI] [PubMed] [Google Scholar]
- Seifert KA, Hughes SJ, Boulay H, et al. 2007. Taxonomy, nomenclature and phylogeny of three cladosporium-like hyphomycetes, Sorocybe resinae, Seifertia azaleae and the Hormoconis anamorph of Amorphotheca resinae. Studies in Mycology 58: 235–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert KA, Morgan-Jones G, Gams W, et al. 2011. The genera of Hyphomycetes. CBS Biodiversity Series 9. CBS-KNAW Fungal Biodiversity Centre, Utrecht, the Netherlands. [Google Scholar]
- Silva BDB, Cabral TS, Marihno P, et al. 2013a. Two new species of Geastrum (Geastraceae, Basidiomycota) found in Brazil. Nova Hedwigia 96: 445–456. [Google Scholar]
- Silva M, Freitas NM, Mendonça HL, et al. 2013b. First report of Stagonosporopsis curcubitacearum causing fruit rot of Luffa cylindrica in Brazil. Plant Disease 97: 1120. [DOI] [PubMed] [Google Scholar]
- Smith ME, Henkel TW, Rollins JA. 2015. How many fungi make sclerotia? Fungal Ecology 13: 211–220. [Google Scholar]
- Smith ME, Pfister DH. 2009. Tuberculate ectomycorrhizae of angiosperms: the interaction between Boletus rubropunctus (Boletaceae) and Quercus species (Fagaceae) in the United States and Mexico. American Journal of Botany 96: 1665–1675. [DOI] [PubMed] [Google Scholar]
- Spegazzini CL. 1910. Fungi Chilensis. Contribución al Estudio de los Hongos Chilenos. Revista de la Facultad de Agronomía y Veterinaria, Universidad Nacional de La Plata 6: 1–205. [Google Scholar]
- Stamatakis A. 2006. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22: 2688–2690. [DOI] [PubMed] [Google Scholar]
- Stamatakis A. 2014. RAxML version 8: A toll for phylogenetic analysis and post-analysis of large phylogenenies. Bioinformatic 30: 1312–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis A, Hoover P, Rougemont J. 2008. A rapid bootstrap algorithm for the RAxML Web servers. Systematic Biology 57: 758–771. [DOI] [PubMed] [Google Scholar]
- Stewart JE, Turner AN, Brewer MT. 2015. Evolutionary history and variation in host range of three Stagonosporopsis species causing gummy stem blight of cucurbits. Fungal Biology 119: 370–382. [DOI] [PubMed] [Google Scholar]
- Su CH, Wang HH. 1986. Phytocordyceps, a new genus of the Clavicipitaceae. Mycotaxon 26: 337–344. [Google Scholar]
- Su HY, Hyde KD, Maharachchikumbura SSN, et al. 2016. The families Distoseptisporaceae fam. nov., Kirschsteiniotheliaceae, Sporidesmiaceae and Torulaceae, with new species from freshwater in Yunnan Province, China. Fungal Diversity 80: 375–409. [Google Scholar]
- Suija A, Motiejūnaitė J. 2017. Calycina alstrupii sp. nov. (Pezizellaceae, Helotiales), a new lichenicolous fungus from Norway. Phytotaxa 307: 113–122. [Google Scholar]
- Sung GH, Hywel-Jones NL, Sung JM, et al. 2007. Phylogenetic classification of Cordyceps and the clavicipitaceous fungi. Studies in Mycology 57: 5–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunhede S. 1989. Geastraceae (Basidiomycotina): Morphology, ecology and systematics with special emphasis on the North European species. Synopsis Fungorum 1: 1–534. [Google Scholar]
- Sutton BC. 1980. The Coelomycetes. Fungi Imperfecti with Pycnidia, Acervuli and Stromata. Commonwealth Mycological Institute, Kew. [Google Scholar]
- Swofford DL. 2003. PAUP*. Phylogenetic analysis using parsimony and other methods. Version 4. Sinauer Associates, Sunderland, Massachusetts. [Google Scholar]
- Tamura K, Stecher G, Peterson D, et al. 2013. MEGA6: Molecular evolutionary genetics analysis version 6.0. Molecular Biology and Evolution 30: 2725–2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K, Hirayama K, Yonezawa H, et al. 2015. Revision of the Massarineae (Pleosporales, Dothideomycetes). Studies in Mycology 82: 75–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsui CKM, Sivichai S, Berbee ML. 2006. Molecular systematics of Helicoma, Helicomyces and Helicosporium and their teleomorphs inferred from rDNA sequences. Mycologia 98: 94–104. [DOI] [PubMed] [Google Scholar]
- Vaghefi N, Pethybridge SJ, Ford R, et al. 2012. Stagonosporopsis spp. associated with ray blight disease of Asteraceae. Australasian Plant Pathology 41: 675–686. [Google Scholar]
- Valenzuela-Lopez N, Cano-Lira JF, Guarro J, et al. 2018. Coelomycetous Dothideomycetes with emphasis on the families Cucurbitariaceae and Didymellaceae. Studies in Mycology 90: 1–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Aa HA. 1973. Studies in Phyllosticta I. Studies in Mycology 5: 1–110. [Google Scholar]
- Vasutová M, Antonin V, Urban A. 2008. Phylogenetic studies in Psathyrella focusing on sections Pennatae and Spadiceae – new evidence for the paraphyly of the genus. Mycological Research 112: 1153–1164. [DOI] [PubMed] [Google Scholar]
- Vellinga EC. 1990. Pluteus. In: Bas C, Kuyper ThW, Noordeloos ME, et al. (eds), Flora Agaricina Neerlandica, vol 2: 31–55. Balkema, Rotterdam. [Google Scholar]
- Verkley GJM, Quaedvlieg W, Shin HD, et al. 2013. A new approach to species delimitation in Septoria. Studies in Mycology 75: 213–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal JM, Alvarado P, Loizides M, et al. 2019. A phylogenetic and taxonomic revision of sequestrate Russulaceae in Mediterranean and temperate Europe. Persoonia 42: 127–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Videira SIR, Groenewald JZ, Nakashima C, et al. 2017. Mycosphaerellaceae – chaos or clarity? Studies in Mycology 87: 257–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visagie CM, Houbraken J, Frisvad JC, et al. 2014. Identification and nomenclature of the genus Penicillium. Studies in Mycology 78: 343–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivas M, Silveira SF, Vivas JMS, et al. 2014. Seleção de progênies femininas de mamoeiro para resistência a mancha-de-phoma via modelos mistos. Bragantia 73: 446–450. [Google Scholar]
- Voglmayr H, Aguirre-Hudson MB, Wagner HG, et al. 2019. Lichens or endophytes? The enigmatic genus Leptosillia in the Leptosilliaceae fam. nov. (Xylariales), and Furfurella gen. nov. (Delonicicolaceae). Persoonia 42: 228–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu D, Groenewald M, De Vries M, et al. 2019. Large-scale generation and analysis of filamentous fungal DNA barcodes boosts coverage for kingdom fungi and reveals thresholds for fungal species and higher taxon delimitation. Studies in Mycology 92: 135–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanasinghe DN, Hyde KD, Jeewon R, et al. 2017. Phylogenetic revision of Camarosporium (Pleosporineae, Dothideomycetes) and allied genera. Studies in Mycology 87: 207–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Binder M, Schoch CL, et al. 2006. Evolution of helotialean fungi (Leotiomycetes, Pezizomycotina): A nuclear rDNA phylogeny. Molecular Phylogenetics and Evolution 41: 295–312. [DOI] [PubMed] [Google Scholar]
- Watling R, Milne J. 2006. A new species of Boletopsis associated with Pinus sylvestris L. in Scotland. Botanical Journal of Scotland 58: 81–92. [Google Scholar]
- Wu G, Li YC, Zhu XT, et al. 2016. One hundred noteworthy boletes from China. Fungal Diversity 81: 25–188. [Google Scholar]
- Yang H, Chomnunti P, Ariyawansa HA, et al. 2014. The genus Phaeosaccardinula (Chaetothyriales) from Yunnan, China, introducing two new species. Chiang Mai Journal of Science 41: 873–884. [Google Scholar]
- Yilmaz N, Visagie CM, Houbraken J, et al. 2014. Polyphasic taxonomy of the genus Talaromyces. Studies in Mycology 78: 175–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousaf N, Kreisel H, Khalid AN. 2013. Bovista himalaica sp. nov. (gasteroid fungi; Basidiomycetes) from Pakistan. Mycological Progress 12: 569–574. [Google Scholar]
- Zalar P, De Hoog GS, Schroers H-J, et al. 2007. Phylogeny and ecology of the ubiquitous saprobe Cladosporium sphaerospermum, with descriptions of seven new species from hypersaline environments. Studies in Mycology 58: 157–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamora JC, Calonge FD, Hosaka K, et al. 2014. Systematics of the genus Geastrum (Fungi: Basidiomycota) revisited. Taxon 63: 477–497. [Google Scholar]
- Zamora JC, Calonge FD, Martín MP. 2015. Integrative taxonomy reveals an unexpected diversity in Geastrum section Geastrum (Geastrales, Basidiomycota). Persoonia 34: 130–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamora JC, Dios MM, Moreno G. 2017. Clarifying the identity of Geastrum campestre var. famatinum (Geastrales, Basidiomycota). Phytotaxa 328: 159–166. [Google Scholar]
- Zhang H, Brankovics B, Luo W, et al. 2016. Crops are a main driver for spe-cies diversity and the toxigenic potential of Fusarium isolates in maize ears in China. World Mycotoxin Journal 9: 701–715. [Google Scholar]
- Zucconi L, Onofri S. 1989. Gyrothrix ramosa sp. nov. and notes on G. citricola. Mycological Research 92: 380–382. [Google Scholar]
- Zwickl DJ. 2006. Genetic algorithm approaches for the phylogenetic analysis of large biological sequence datasets under the maximum likelihood criterion. PhD thesis. University of Texas, Austin, USA. [Google Scholar]