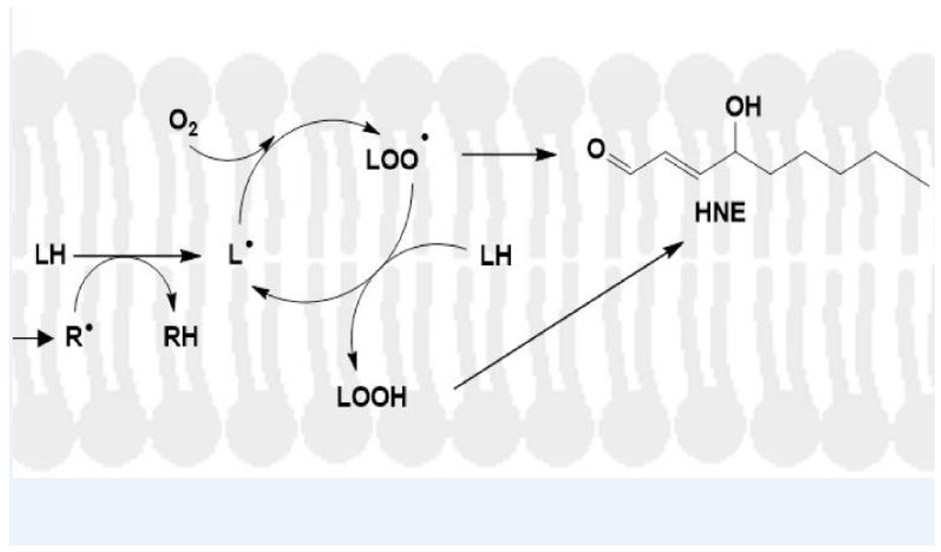
Figure 3.
Mechanism of lipid peroxidation. A free radical, for example associated with Aβ42 oligomers inserted into a lipid bilayer (Butterfield and Halliwell, 2019), attacks a lipid acyl chain-resident, labile allylic hydrogen atom to form the RH compound and a carbon-centered lipid free radical, L.. Oxygen, which is paramagnetic with two unpaired electrons and has zero dipole moment, meaning it is highly soluble in the lipid bilayer, binds to the carbon-centered radical on the acyl chain to form the lipid peroxyl free radical, LOO.. This free radical, in turn, abstracts another allylic hydrogen on a fatty acid chain of the lipid to from LOOH, the lipid hydroperoxide, and another lipid-resident, carbon-centered free radical, i.e., propagating the chain reaction. LOOH, through a series of chemical reactions, spontaneously will form HNE that can covalently bind to and change the conformation and function of membrane-resident proteins (Subramaniam et al., 1997; Sultana et al., 2013). In addition, HNE can diffuse from the lipid bilayer to covalently modify cytosolic proteins (Butterfield and Stadtman, 1997). Modified from Butterfield and Boyd-Kimball, 2019.