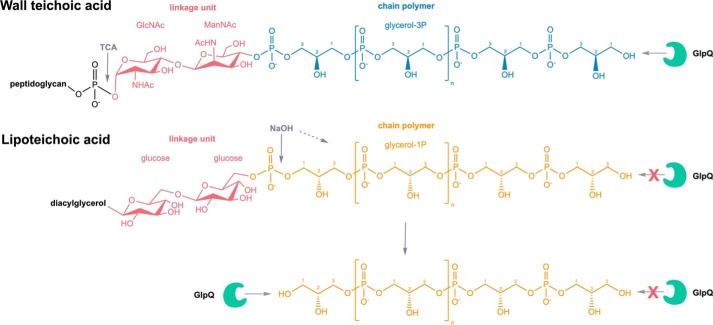
Figure 8.
Differential digestion of WTA and LTA by the stereospecific sn-glycerol-3P phosphodiesterase GlpQ. WTA of B. subtilis 168 are phosphodiester polymers made of Gro3P subunits that are generally substituted at the hydroxyl group at the C2 position to a certain degree with d-alanine or alpha-glucose. Via a linkage unit (red) consisting of a disaccharide, ManNAc-β(1–4)-GlcNAc, and an unsubstituted Gro3P, WTA are linked to the C6 of N-acetylmuramic acid of the peptidoglycan via a phosphodiester bond. Trichloroacetic acid (TCA) treatment enables cleavage of the glycosidic phosphodiester bond that connects the WTA linker with the PGN. LTA of B. subtilis 168 is a phosphodiester polymer made of Gro1P subunits; hence, it represents an enantiomer of the WTA polymer. It can be modified at the C2 position with d-alanine or GlcNAc and linked to a diacylglycerol via a glucose-β-1,6-glucose linker disaccharide. GlpQ is able to cleave off Gro3P from the terminal ends of WTA. Conversely, GlpQ is not able to chip off Gro1P from the terminal ends of LTA. The stereospecific enzyme GlpQ is able to discriminate the orientation of the hydroxyl group on the C2. Treatment with NaOH enables precleavage of phosphodiester bonds within the LTA chain polymer, resulting in fragments that contain Gro3P terminal ends. From these ends, GlpQ is able to cleave off Gro3P moieties. The differential cleavage of WTA and LTA by GlpQ unravels the different stereochemistry of the polymers.