Figure 5.
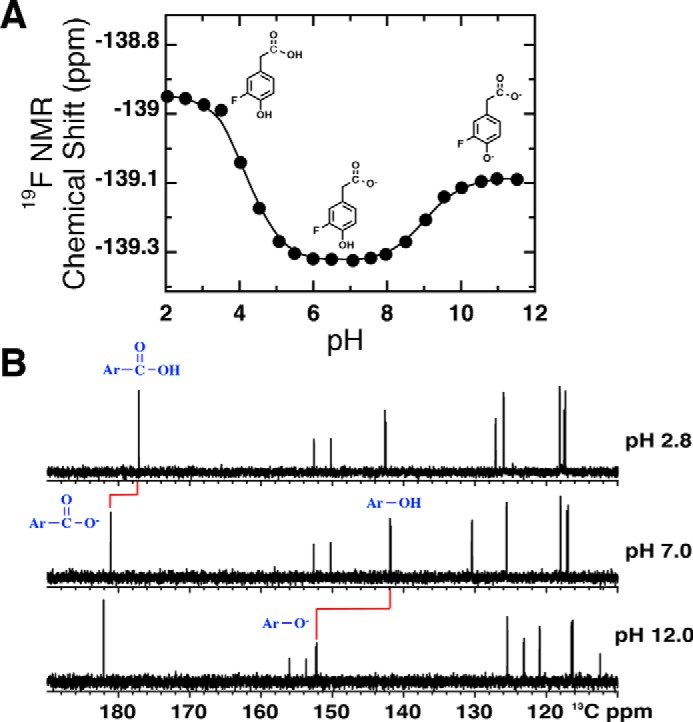
pH titration curves of 3F4HPA in the absence of WT C2 at 25 °C. A, pH dependence of the 19F chemical shift of 3F4HPA. Fitting to the Henderson-Hasselbalch equation (Equation 3) yielded pKa values of 4.2 ± 0.04 and 9.0 ± 0.02 for the carboxyl and hydroxyl group, respectively. Structures of the ionization states of 3F4HPA dominating in the three pH regions are shown, based on the data in B. B, 13C NMR determination of the events responsible for the different pKa values. The carboxyl carbon near 180 ppm is most sensitive to the pKa of 4.2, and the phenolic carbon near 140 ppm (at pH 7.0) is most sensitive to the pKa of 9.0.
