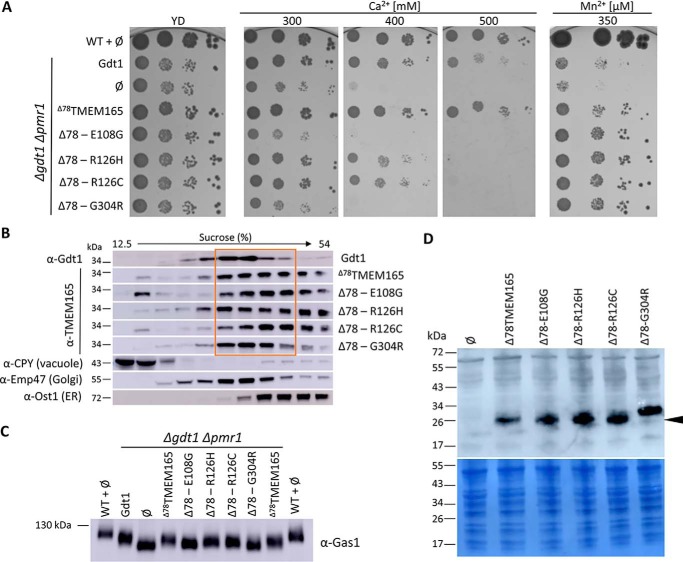
Figure 6.
A, phenotypic effects of Δ78TMEM165-CDG-related mutations within gdt1Δpmr1Δ strain. The gdt1Δpmr1Δ strain expressed GDT1, Δ78TMEM165 truncated version, or Δ78TMEM165 containing CDG-causing mutations. ∅, gdt1Δpmr1Δ with the empty pRS416 vector. The cells were treated as described in the legend to Fig. 1B. B, subcellular fractionation of gdt1Δpmr1Δ strain expressing GDT1, Δ78TMEM165, or mutated Δ78TMEM165. Each strain was lysed and fractionated on a sucrose gradient. The collected fractions were analyzed by Western blotting using antibodies against Gdt1 or TMEM165 and against markers of the different subcellular compartments (CPY, Emp47, and Ost1). The distributions of the organellar markers shown here are representatives of those observed in all the strains. C, glycosylation of Gas1p. Total membrane proteins were extracted from the WT strain or gdtlΔpmr1Δ strains expressing GDT1, Δ78TMEM165 truncated version, or Δ78TMEM165 containing CDG-causing mutations. The cells were grown in MD-U supplemented with 200 mm CaCl2 to an A600 of 3. The levels of Gas1p were analyzed by Western blotting with anti-Gas1 antibodies. D, production of the different TMEM165 versions in gdtlΔpmr1Δ strain. Total protein extracts of cells grown in MD-U medium to an A600 of 3 were analyzed by Western blotting with antibodies directed against TMEM165.