Graphical abstract
Abbreviations: ABS, Acrylonitrile butadiene styrene; PLA, Polylactic acid
Keywords: Stereotactic surgery, Craniotomy, Electrophysiology, Intracranial pharmacology, Optogenetics, Brain model
Highlights
-
•
Accessible, cost-effective brain model for training in surgical techniques.
-
•
Flexible debugging tool for optimizing noise levels, etc. of a new experiment.
-
•
Training in electrophysiology, intracranial pharmacology, and optogenetics.
-
•
Realistic experience close to real stereotactic surgeries.
-
•
The model can be modified to fit individual lab equipment and goals.
Abstract
Background
In accordance with the three R principles of research, animal usage should be limited as much as possible. Especially for the training of entry-level scientists in surgical techniques underlying opto- and electrophysiology, alternative training tools are required before moving on to live animals. We have developed a cost-effective rat brain model for training a wide range of surgical techniques, including, but not limited to optogenetics, electrophysiology, and intracranial pharmacological treatments.
Results
Our brain model creates a realistic training experience in animal surgery. The success of the surgeries (e.g. implantation accuracy) is readily assessable in cross sections of the model brain. Moreover, the model allows practicing electrophysiological recordings as well as testing for movement or light related artefacts.
Comparison with Existing Method(s)
The surgery and recording experience in our model closely resembles that in an actual rat in terms of the necessary techniques, considerations and time span. A few differences to an actual rat brain slightly reduce the difficulty in our model compared to a live animal. Thus, entry level scientists can first learn basic techniques in our model before moving on to the slightly more complex procedures in live animals.
Conclusions
Our brain model is a useful training tool to equip scientist who are new in the field of electrophysiology and optogenetic manipulations with a basic skill set before applying it in live animals. It can be adapted to fit the desired training content or even to serve in testing and optimizing new lab equipment for more senior scientists.
1. Introduction
A large proportion of research targeting neurophysiology relies on in vivo animal experiments, utilizing a plethora of techniques such as electrophysiology, circuit tracing, and optogenetic and pharmacological manipulations. While conducting these experiments in a live organism is inevitable to study neuronal processes, preparatory steps can be conducted in alternative models. Broadly, two types of instances can be distinguished, i.e. (1) the training of entrance level scientists and (2) establishing a new in vivo experiment. Conducting stereotactic craniotomies, virus injections and implantations requires not only fine motoric skills but also knowledge of how to use the equipment (e.g. an intuition of the degrees of freedom of stereotactic arms) and how to handle delicate probes (e.g. electrophysiological electrodes). With our model, all of those procedures can be trained without an animal. Further, for setting up a new neurophysiological in vivo experiment, functionality and noise levels of the setup need to be tested, which includes both individual tools such as electrodes, fibers and recording devices, as well as aspects of the behavioral setup or arena used for recordings. Even if individual tools or procedures have been applied successfully in the lab, unanticipated complications can arise from a newly assembled setup. Here, we provide a model for testing caveats of the desired setup. As our model shares the required properties such as electrical conductivity and light spread for electrophysiological and optogenetic experiments, respectively, it can yield the same information about a setup’s basic viability as a live brain.
In line with the three R’s of research, Replace, Reduce and Refine (Russell and Burch, 1959; Tannenbaum and Bennett, 2015; Kirk, 2018), we propose to replace live animals with an alternative for training and establishing intracranial injections, stereotactic implantations, and extra cellular recordings. The challenge in implementing the first of the three R’s, Replace, lies in the search for a viable alternative, which is sufficiently similar to a live brain to conduct a given experiment. For pharmacological treatments and anesthesia, both computer models and dry models have been developed (Dewhurst, 2004; Dewhurst et al., 1994; Hughes, 2001; Schmidt et al., 2011; Stevens and Dey, 2007). For training of in vivo neuroscientific experiments, we propose three different models of different degree of realism and accessibility: a 3D printed skull, the plastic beaker model, and an agar block. Based on these models we describe how to train specific procedures such as craniotomies, duratomy, extracellular recordings, intracranial injections and how to use those models to optimize the experimental setup with regards to noise sources for extracellular recordings, light distribution and photoelectric artefacts during optogenetic experiments.
2. Materials and method
2.1. Assembling and preparing the model brain
The model brain is conceptualized such that its assembly can be accommodated in any lab. Its three major components are a skull model, an electrically conductive solution (milk or saline), a thickener (agar), and a light diffusing and absorbing substance (milk). For the outer shell, either a 3D printed skull or a simplified model in the form of e.g. a plastic beaker can be employed, depending on available equipment and envisioned application. We modified and supplemented the 3D model developed by Artem Kutikov (https://www.thingiverse.com/thing:469391/files) to include placeholders for muscles, ear-bar simulation support, lambda and bregma defining sutures, opening in the base of the skull such that artificial dura and agar could be inserted, and finally all small foramina were closed such that the skull could be filled with agar. The final 3D model can be downloaded (https://www.thingiverse.com/thing:4042410, https://github.com/Optophys/Agar-brain). We printed the skull with a Ultimaker 3 3D printer (Ultimaker, Netherlands), using ABS plastic filament (White Ultimaker ABS Filament). In comparison to PLA, the ABS filament has a high melting point which allows high speed drilling during the generation of craniotomies.
To assemble the skull, it was first turned upside down such that artificial dura, blood vessels and brain matter could be added. For the brain itself, we chose a milk solution thickened with agarose. Milk adds electrical conductivity to the brain solution due to its soluble salt fraction (Lanzanova et al., 1993), while also providing a similar light refraction to an actual brain (Pogue and Patterson, 2006). Alternatively, a saline solution could be used to have more control over the degree of electrical conductivity. In the here reported case, we mixed 0.5 mg of agar with 50 ml 1.5 % fat milk and heated the mixture up to boil in a microwave three consecutive times. The solution was then slowly cooled down to room temperature under running water. Before filling the solution into the skull, a piece of Parafilm (Bemis Company, USA), was placed in the cavity of the skull. To avoid that the parafilm slides during duratomy, the parafilm should be sufficiently large such that it fits tightly within the 3d skull. Although the Parafilm is more flexible than the dura matter, the same tools and techniques can be used for its removal as for the dura matter. The artificial dura also simulates that the exposed brain area might be smaller than anticipated due to the remainings of the dura-matter surrounding the opening. This is crucial for planning the right craniotomy size for the implantation of electrodes and fibers. Additionally, blood vessels can be modelled by putting red plastic thread (Synthetic clown hair) ventral of the Parafilm. In order to ensure its attachment to the skull and the avoidance of hollow spaces, the Parafilm should be pressed down firmly throughout the addition of the agar solution. It is also advisable to begin with a few drops of agar solution and letting them solidify before adding the rest. When using a plastic beaker, the agar solution should extend slightly beyond the beaker to ensure its adherence to the skull when turning it later. After five to ten minutes, the agar solution becomes sufficiently solid to start the surgery. To maximize stability, the plastic beaker should be attached to the stereotactic frame plate using adhesive film.
When utilizing the 3D printed skull, the dents in the location of the ear canals and curvature of the teeths can be used to place the skull in a three point fixed manner into a stereotactic frame. For practicing ear bar placement, the skin outside the ear canal was modeled with a 20 × 10 mm rubber sheet from a dermatril glove (Art. Nr. 740, KCL, Germany) and attached with a simple packaging foam. The marked locations of lambda and bregma aid targeting specific coordinates for the craniotomy. In both cases, craniotomy and duratomy can be performed with a drill and forceps. Upon reaching the agar brain surface, one or all of the following techniques can be performed.
2.2. Electrodes
The proposed model employs simple electrodes, which can be assembled without extensive prior training. In theory, a single electrode can suffice, but electrode arrays yield more reliable information, particularly when testing a new experimental setup. A simple array can be built using a Mill-Max connector (Mill-Max, Oyster Bay, USA), two to four pieces of tungsten wire (30 μm, polyimide insulation, WHS Sondermetalle, Grünsfeld, Germany), a plastic holder and, if possible, guide tubes (TSP075200, Polymicro, Molex, USA). The Mill-Max connector was glued on the top part of the plastic holder with cyanoacrylate (“Super Glue”), while the guide tubes were glued to the lower part of the plastic holder. Tungsten wires were then placed through the guide tubes. Leaving one tube empty in between ensures equal distances between them. The wires were inserted into the openings of the Mill-Max connector and secured with pins (pins were first removed from the Mill-Max connector) to deinsulate the wires and to ensure an electrical connection between pin and electrode wire. Having added all wires, they were glued to the guide tubes with cyanoacrylate and cut to the desired length at the lower end of the electrode. If no guide tubes are available, they can be glued directly to the plastic holder. For the reference electrode, either stainless steel or silver wire can be soldered onto another 2 × 2 Mill-Max connector. The electrode impedances were determined with an impedance meter (IMP-2A-MC 18 Channel, Microprobes, Gaithersburg, USA).
After craniotomy and duratomy, the recording electrode was placed at the desired depth using a stereotactic frame. In a training context, closing the craniotomy can be practiced by first applying Kwik-Cast (WPI, Sarasota, FL, USA) to protect the brain and subsequently dental cement (Paladur, Kulzer GmbH, Hanau, Germany) to stabilize the guide tubes. The plastic holder can then be melted off upward of the guide tubes as well as around the Mill-Max connector using a electrocauterizer (Bovie, WPI, Germany). The Mill-Max connector was fixed to the skull anterior to the desired cross section axis using dental cement. The reference electrode was wrapped around a screw placed in the skull over another brain region such that the screw touches the agar brain. Dental cement was again used to fix the Mill-Max connector.
2.3. Noise testing
Upon electrode implantation, noise testing of the setup can be performed, which is equally useful in training and equipment testing situations. Both the recording and reference electrodes were connected to a recording system with an amplifier (in our case RZ5D, Tucker Davis Technologies, USA but any electrophysiology system would work). In addition to baseline noise levels, noise and artefacts from various sources can be recorded, depending on the envisioned experiment or training content. Here we tested the model inside and outside of a Faraday cage, an grounded and ungrounded Faraday cage, a grounded and ungrounded recording model, the addition of power supplies, and flexible cables or fixed connector to preamplifier.
2.4. Simulated in-vivo extracellular recordings
For the extracellular stimulation we used the Stimsola stimulator (BioPac Systems Inc., Goleta, USA) to inject a user defined current into the plastic beaker model. To this end we used the CurrentMode and 1Kohm setting. The stimulation wires (standard steel wires) were placed at a distance of 10 mm from the recording electrode (tungsten wires as described above). We outputted a broadband extracellular recording with a maximal current of 80 μA. This generated an extracellular potential of around 600 μV sampling rate of 30000 Hz. To detect spikes we used a threshold of −250 μV. For sorting the spikes we cut out −10 to 40 samples (51 samples in total) around each spike. The first two principal components were used for identifying separable clusters. Spike waveform variability was quantified in terms of the standard deviation across spikes at each time point.
2.5. Injections
Pressure injections for pharmacological and optogenetic experiments were performed using 10 μl gas-tight Hamilton syringe (World Precision Instruments, Sarasota, FL, USA) and glass capillaries (Z611239-250EA, Hirschmann, Eberstadt Germany) were pulled horizontally (p87, Sutter Instrument, CA, USA). In order to follow the spread, substances with distinct colors are preferable. Here we used edding color (Refill ink, T25, Color 9, Edding, Germany) for the injection in the 3D printed skull and blue ink (Refill ink, T100, Color 3, Edding, Germany) for practice injections in clear agar. In the following, 1 μl of a 1:10 times dilution of either T25 or T100 was injected over 10 min.
2.6. Light testing and full implantation and recording in 3D printed skull
For optogenetic experiments, optical fibers were assembled in house. FT 200 EMT fibers (Thorlabs) with a diameter of 200 μm were connected to a ferrule with a diameter of 2.5 mm with a bore of 230−240 μm using Epoxy (ND-355, EpoTek, Germany) according to manufacturer’s protocol. After heat curing the epoxy with a heat gun (HL 1710s, Steinel, Herzebrock-Clarholz, Germany) the fiber end was polished using polishing paper (LF5P, LF1P, and LF03 P, Thorlabs, Dachau, Germany), and inspected using a fiberscope (FS200, Thorlabs, Dachau, Germany). Finally, the fiber was shortened to the desired length of 6 mm, and was implanted and fixated with dental cement and silicon. If implanted together with an electrode to record photoelectric artefacts, an angle of 45–60 degrees should be kept in between fiber and electrode to promote a measurable artefact. To guarantee a light artefact in the case of training purposes the insulation can be removed at the tip of the wire by scraping with a scalpel. Yellow light with a wavelength of 594 nm or blue light with a wavelength of 475 nm was delivered in 5 ms pulses at 20 mW from the fiber tip.
Electrode implantation, injection and optical fiber were conducted in the same 3D printed rat skull. Photoelectric artefacts were recorded in the complete model as well as after a cross section to visualize the interplay of the individual components. The cross section was made using a custom built plastic cutter. To this end, a potential of 3 V was applied across a 3 cm long nichrome wire of 200 μm diameter (34,7 O/m, Ni 80 % and Cr 20 %). A smaller diameter of 120 μm typically resulted in a broken wire and cannot be recommended.
3. Results
3.1. Model brain
The brain model is easily assembled with low-cost, commonly available materials. Ideally a 3D printer is at hand, such that a rat skull replica can be printed (Fig. 1A–C). The 3D printed model skull allows practicing the positioning in a stereotactic frame (Fig. 1D). A stable three point fixation is ensured by the front-teeths and the two ear channels. The ear channel cannot be seen through the latex membrane but had to be felt with the tip of the ear bars through the latex membrane, thus mimicking the challenges inherent in a three-point fixation. At the same time, the absence of skin and fur still allows a more intuitive grasp of the general bone structure than in an intact animal. As an alternative, if no 3D printer is at hand, the simple plastic beaker provided a faithful model of many aspects of the surgery (Fig. 1E). Both options serve for low-stakes equipment testing as well as animal-free craniotomy and duratomy training and can be adapted to the desired application and the available equipment in lab.
Fig. 1.
Assembling the model brain.A: Plastic skull model and material for brain and ear canal model. B: Building the artificial ear channel: For practicing the insertion of ear bars, a piece of a latex glove (blue) was squeezed against the ear channel using foam plastic (black). C: The individual steps of filling the skull with the agar brain solution. Parafilm and the threads mimicking blood vessels are placed in the skull, followed by a few drops of semi cooled down agar solution. Only after the initial agar layer has solidified, the skull is entirely filled with agar. D: Three point fixation of the plastic skull in the stereotactic frame. E: Assembling the plastic beaker model.
3.2. Craniotomy and duratomy
During the craniotomy, the haptic feedback from the drill is similar to a real skull, which enables the acquisition of drilling techniques. This is true for both the skull model (Fig. 2A) as well as for the plastic beaker model (Fig. 2B). While thinning the skull, the plastic becomes elastic similar to a trampoline and realistically translucent upon approaching the dura mater, thus providing visual feedback. Furthermore, similar to a real craniotomy when the bone cracks water protuded.
Fig. 2.
Craniotomy and duratomy. A, B: Stereotactic craniotomy and duratomy on the skull model and plastic beaker, respectively.
3.3. Assembly of electrodes and fibers
Here we used electrodes that enables the targeting of multiple brain areas (Fig. 3A and B). The two shanks of the stair case electrodes can be separated by melting the plastic such that the two shanks can be separated and placed in different locations. This is particularly usefull in order to avoid blood wessels and for conducting pilot experiments. With this approach, we have successfully implanted 44 electrodes divided across eight shanks using this type of electrodes. The tungsten wires had impedances ranging between 200–500 kOhm at 1 kHz (Fig. 3C). The optical fiber was assembled using standard components (Fig. 3D). Crucial steps are adding epoxy and heat curing the assembly (Fig. 3E) such that the inlet fiber surface can be polished (Fig. 3F).
Fig. 3.
Assembling electrodes and fibers. A: Electrode materials and assembly. The thin plastic from a laboratory beaker was used as the electrode substrate. Guidetubes and connectors for electrode wires were glued to the electrode substrate. Electrode wires were inserted in the guide tubes and in the connector. Finally connector pins were inserted, electrode wires were glued to the guide tubes and were cut to the correct length. B: A staircase structure allows one pair of electrodes (left plastic arm) to be inserted first in one location and one pair of electrodes (right plastic arm) to inserted later in another location (left panel). Magnification of guide tubes and electrode wires (right panel). C: Measurement of electrode impedances in saline via an impedance meter. D: Fiber materials and assembly. The protective buffer (blue) was removed from the optic fiber and was inserted into an metal ferrule. E: The ferrule is filled with epoxy before the fiber is inserted (left and middle panel). The optical fiber was fixated to the ferrule by heat cured epoxy (right panel). F: The quality of the polishing of the optical fiber (left panels) was tested with a fiber scope. A good light inlet shows a surface free of scratches (middle column and top row) and a full moon of light transmission (middle column and bottom row). A suboptimal light inlet shows a dirty surface (right column and top row) and low light transmission (right column and bottom row).
3.4. Electrode implantation
Electrode implantation could be practiced in terms of the limited space within the craniotomy, electrode angle, reference electrode and connector location for the skull model (Fig. 4A–C) as well as for the plastic beaker (Fig. 4D). Furthermore the addition of artificial blood wessels brings the experience closer to reality as care needs to be taken to avoid them. Although, the missing muscle tissue on top of the skull is replaced by a rim of plastic to provide a realistic space for cementing connectors, the absence of skin flaps to aid in cement smoothing and forming made the model experience slightly more difficult than in-vivo. As for the reference electrode, its placement via a screw placed over an area far from the recording area could be trained as in a real surgery.
Fig. 4.
Electrode implantation. A-C: Electrode implantation in a skull model. A: Electrode reference implantation. B: Electrode wire implantation. C: Releasing electrode shank from holder by melting plastic. D: Electrode implantation in a plastic beaker.
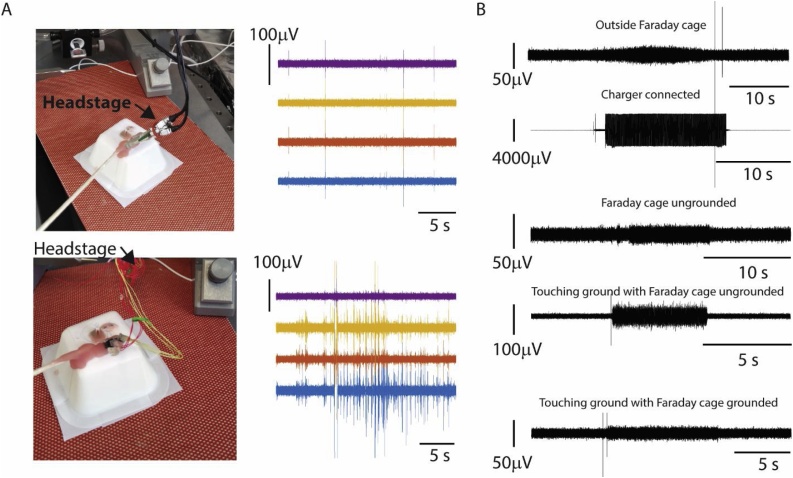
3.5. Noise testing
For the noise testing we recorded from the plastic beaker model using a Tucker Davis Technologies recording system. Electrodes that are connected with flexible cables to the impedance converting stage (head stage) are prone to movement artefacts. Indeed, strong artefacts occurred during movements of flexible cables between electrode connector and impedance conversion (Fig. 5A). Even for a cable-free connection between electrodes and impedance conversion, we observed movement related artefacts. Those artefacts are partially due to the moving cables and partially due to the movement within the zif-connector which illustrates the advantage of digitizing the signal before sending it over a connection (Du et al., 2011; Jun et al., 2017). Furthermore, we tested noise originating from recording outside a Faraday cage, leaving a nearby charger of the pre-amplifier connected, an ungrounded Faraday cage, and the agar of the model brain touching the ground with the Faraday cage being ungrounded and grounded, respectively (Fig. 5B). The induced fluctuations in the recorded signal ranged between 25 μV–500 μV. In each instance, the induced noise could clearly be distinguished from baseline level, thus making our model a valuable tool for noise testing of a new scientific setup as well as training basic electrophysiology.
Fig. 5.
Noise testing. A: Noise testing setup. Increase of artefacts caused by loose cables between the electrodes and impedance conversion (bottom row) in comparison to a direct connection between electrodes and impedance conversion (top row). B: Quantitative results from noise testing comparing the impact of a Faraday cage as well as a connected charger of the pre-amplifyier.
3.6. Simulated in-vivo extracellular recordings
To simulate in-vivo extracellular recordings we injected a time varying current originating from a previous in-vivo extracellular recording to the plastic beaker model (Fig. 6A and B). The current stimulation generated an almost identical potential trace in the agar (Fig. 6C). Similarly to a real in-vivo recording we high-pass filtered (cut off 300 Hz) the extracellular potential (Fig. 6D) and sorted the spikes into units (Fig. 6E and F). The separable clusters render this approach a suitable model for illustrating spike sorting of extracellular recordings.
Fig. 6.
Realistic extracellular recordings in a plastic beaker model. A: Experimental setup. A current generator was used to generate a realistic extracellular signal. B: The injected extracellular current. C: The potential recorded in agar during current injection. D: High-pass filtered potential recorded in agar. E: The first two principal components of the detected spikes. Two well separable clusters (orange and blue) and two multiunit clusters (red and green) were manually selected. F: Average waveforms for the four spike clusters marked in E. The shaded area corresponds to the standard deviation.
3.7. Injections
Injections in the agarose brain was successfully performed using either Hamilton syringes or glass pipettes. When using glass pipettes, care needs to be taken to avoid clogged pipette tips by either replacing or cleaning them with ethanol after 2–5 injections. The outcome of the agar injection provides a direct feedback about the quality of the pulled pipettes. Further, the amount of backflow (i.e. the upward spread of injected fluid along the injection track) can be characterized. In the here provided example of ink injected in agar, glass pipettes were more prone to backflow (Fig. 7A). A Hamilton syringe used with the same volume and injection depth avoided major backflow (Fig. 7B). The direct illustration of backflow emphasizes the relation between recording depth, injection volume and injection duration. Finally the injections may be practiced in the plastic beaker model or the skull model (Fig. 7C). Injection in the skull model provides training in stereotactic procedures such as lambda-bregma leveling and bregma referencing.
Fig. 7.
Injection of ink. A-B: Injections in a transparent agar block with either a pulled glass pipette (A) or a Hamilton syringe (B). C: Stereotactical injections in a skull model (left). The ejection of a small drop before injection (right) ensures that the pipette is not clogged (middle).
3.8. Light testing and recording in the skull model
Next we tested fiber implantation, laser stimulation and photo electric artefacts with the skull model. Craniotomy, duratomy, injection, and electrode implantation were conducted beforehand as described in the previous paragraphs. We stereotactically positioned the fiber and applied Kwik-Cast and dental cement to secure the implanted fiber. Electrode recordings paired with optical stimulation during fiber implantation demonstrated a classic photoelectric artefact (Fig. 8A). To better visualize the finalized implantation (Fig. 8B), we performed a cross section with a heat wire tool (see methods) (Fig. 8C). The injected pink ink surrounded the electrodes (Fig. 8D). Optical stimulation both at 475 nm and 594 nm produced photoelectric artefacts (Fig. 8E). Although yellow light has a substantially larger spread than blue light, both are clearly visible in the cross section. For training purposes, these cross sections could be used as an assessment of the implantation quality. For example, targeted versus widespread light delivery or overlap of injection and fiber placement can be tested.
Fig. 8.
Ilumination tests, full implantation and recordings in 3D printed skull. A: Optical fiber is implanted while it is connected to a light source (left). The electrodes were connected to the recording system (middle) such that the photo electric artefact could be studied during implantation (right). B: The finished implantation. C: A wire cutter (left) was used to make a cross section (right) through the implanted model (middle). D: Cross section showing the injection site. E: Overlap between injection and illuminated brain could be studied for yellow (top) and blue (bottom) light. Photo electrical artefacts for both wavelengths (right). In C and E, the cross section of the model brain was overlaid with an image from a Rat Brain Atlas (2009, George Paxinos and Charles Watson).
4. Discussion
The brain and skull model described here is a versatile starting kit for training in vivo electrophysiology, optogenetics and pharmacology in various degrees of sophistication, depending on available lab equipment. Futher, it can serve to test new lab equipment or setups before starting in vivo experiments. We have demonstrated that the 3D printed skull model enables realistic craniotomy and duratomy training, accessible for entry level scinetists. Alternatively, without access to a 3D printer, a simplified model built around a plastic beaker also conveys crucial surgery skills easily transferred to in vivo settings.
Brain tissue consists of neuronal cell bodies, their processes including dendrites and axons which form either sparse branches or dense fiber bundles, the interconnecting extracellular brain matrix, glial cells, blood vessels, and extracellular fluid. Each of these components as well as their combination may have different influences on the mechanical properties of the tissue, which, in turn, may influence the penetration success of neural probes or the diffusion of viral particles or pharmacological agents (Antonovaite et al., 2018). It is unlikely that all of the biological features can be perfectly mimicked with a simple agarose brain. However, certain aspects can be approximated. The density of an agar brain can be easily calculated as weight per volume (g/cm3) which directly refers to percentages of agar; e.g. an agarose mixture of 1% refers to 0.1 g/cm3 strongly resembling the average density of brain tissue of 0.99 g/cm3 (Beckmann et al., 1999). The exact values vary of course with the exact brain structure of interest. For realistic tests of a probe penetrations, more elaborate measurements of viscoelasticity have to be conducted (Antonovaite et al., 2018). Finally, for the evaluation of diffusions of injected compounds, a direct side-by-side comparison between different agarose mixtures and real brains will result in the most reliable values. This comparison has been conducted for convection enhanced delivery injections indicating that a concentration of 0.6 % (or as in our case 1%) agarose resembles brain tissue the most in terms of diffusion patterns (Chen et al., 2004).
4.1. Extensions of the model
As much as the model provides a realistic training experience, still several differences to real animals are evident, e.g. the abundance of space to cement connectors in the plastic beaker model. The limited space for electrodes, fibers and their respective connectors is a crucial aspect during in vivo implantation. By adding a model of the animal body, this aspect can become more realistic. Second the parafilm is more elastic than the dura mater rendering the duratomy slightly more difficult. To make the experience more realistic, the parafilm could be replaced by an artificial dura (Heo et al., 2016). Further, the described model can easily be extended to include additional training aspects, such as a model of muscle tissue to practice the skull cleaning before craniotomies. Likewise, the 3D printed skull can be fit to match the dimensions of other animal models, such as a mouse or a monkey (Pohl et al., 2013). Similarly it is conceivable that the model can be used to practice implantations for head mounted mini-scopes and to test the imaging system by adding fluorescent particles to the agar. Overall, this model is a valuable starting point, which accommodates various applications.
4.2. Establishing electrophysiology for freely moving animals
Simple, self-assembled electrodes enable a fruitful teaching experience, covering the basics of electric signals, realistic implantation as well as distinguishing noise and artefacts from actual information. While these basic electrophysiological concerns could also be taught in the agarose solution without the surrounding skull model, working through all the methods listed here (assembly of materials, implantation and recording) alongside a single model provides the most cohesive training experience. If a certain problem has been isolated, for example recording or injection, one can revert to simpler models such as the plastic beaker or an agar block for trouble shooting. It should also be noted that both approaches (i.e. skul model and plastic beaker) effectively convey common sources for noise and artefacts, which facilitates the development of precautionary measures to enable a successful in-vivo recording. The most common measurement problems can thus be isolated to validate a new setup or prepare entry level scientists for in vivo experiments. Finally, the techniques presented here allow for the implantion of electrodes at multiple different locations and thus is suited for a pilot experiment also in an in vivo setting. Overall, the noise testing model can be used for establishing a freely moving recording system, including the optimization of connectors and cables as well as the elimination of ground loops.
4.3. Injections
Injections of pharmacological agents or viral vectors can be practiced with the model. For training purposes, using a dilution of off-the-shelf ink is not only cost-effective, but also includes training in dilution preparation. Although the dynamics of the diffusion in agar differs from those in brain tissue, the manual skills underlying a successful injection are sufficiently similar to enable a profitable training experience. Entry level scientists can compare the results obtained with different volumes, dilutions and pipettes and immediately assess the quality of th injections in a brain cross-section. Thus, proper injection techniques can be trained and experimental progress can be monitored promptly. In preparation for optogenetic experiments, even the co-localization of injection and light delivery to the injection site can be practiced, thus preparing for in vivo optogenetic experiments (see Fig. 8D and E).
4.4. Optical stimulation
Both electrode implantations and injections can be combined with light delivery via optical fibers. Fiber assembly can be carried out in lab, including polishing and observation of light spread. During fiber implantation, entry level scientists can practice handling the stereotactic frame to achieve targeted light delivery to the injection site. Practicing such multi-step surgeries in a model prepares for in vivo applications. In electrophysiological recordings, photoelectric artefacts can be demonstrated and the placement of optical fibers relative to electrodes can be optimized.
5. Conclusion
In brief, the presented agar brain model and its variations permit realistic training of in vivo electrophysiology, optogenetics and intracranial pharmacology regardless of lab equipment and skill set. Common surgical techniques as well as the usage of specific scientific equipment can be trained before moving on to in vivo experiments. The lack of time pressure and additional concerns such as anesthesia maintenance and potential bleedings simplify the conduction of craniotomies for entry level scientists. Overall, the model presented here will not only help researchers to become fluent with equipment, tools and complex stereotactic procedures, but will also help researchers to plan and optimize the experimental setup.
The cluster of excellence BrainLinks-BrainTools (EXC 1086) also belongs to the DFG funding. So it shoudl read:
Contributions
D.E. and I.D. designed the study. D.E., M.S. and I.D. wrote the manuscript. D.E. and M.S. conducted the experiments with assistance of P.C. during the injection experiments. A.S. helped optimizing the experiments. D.E. acquired the figures including photos, P.C. and M.S. acquired the photos of Fig. 6 a and b.
Acknowledgments
We would like to thank the students of the Optophysiology course at the University Freiburg for their valuable feedback. This work was supported by Master 2016 funds, the Bernstein Award 2012 (01GQ2301), the cluster of excellence BrainLinks-Brain-Tools (EXC 1086), the Deutsche Forschungsgemeinschaft (DFG) via the cluster of excellence BrainLinks-Brain-Tools (EXC 1086), and the grants DI 1908/3-1 and 1908/6-1, the BW Stiftung (RatTrack), as well as the ERC Starting Grant OptoMotorPath (338041), all to I.D.
References
- Antonovaite N., Beekmans S.V., Hol E.M., Wadman W.J., Iannuzzi D. Regional variations in stiffness in live mouse brain tissue determined by depth-controlled indentation mapping. Sci. Rep. 2018;8(1):12517. doi: 10.1038/s41598-018-31035-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann F., Heise K., Kölsch B., Bonse U., Rajewsky M.F., Bartscher M., Biermann T. Three-dimensional imaging of nerve tissue by x-ray phase-contrast microtomography. Biophys. J. 1999;76(1 Pt 1):98–102. doi: 10.1016/S0006-3495(99)77181-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z.-J., Gillies G.T., Broaddus W.C., Prabhu S.S., Fillmore H., Mitchell R.M., Corwin F.D., Fatouros P.P. A realistic brain tissue phantom for intraparenchymal infusion studies. J. Neurosurg. 2004;101(2):314–322. doi: 10.3171/jns.2004.101.2.0314. [DOI] [PubMed] [Google Scholar]
- Dewhurst D. Computer-based alternatives to using animals in teaching physiology and pharmacology to undergraduate students. Altern. Lab. Anim.: ATLA. 2004;32(Suppl 1B (June)):517–520. doi: 10.1177/026119290403201s83. [DOI] [PubMed] [Google Scholar]
- Dewhurst D.G., Hardcastle J., Hardcastle P.T., Stuart E. Comparison of a computer simulation program and a traditional laboratory practical class for teaching the principles of intestinal absorption. Am. J. Physiol. 1994;267(6 Pt 3):S95–104. doi: 10.1152/advances.1994.267.6.S95. [DOI] [PubMed] [Google Scholar]
- Du J., Blanche T.J., Harrison R.R., Lester H.A., Masmanidis S.C. Multiplexed, High Density Electrophysiology with Nanofabricated Neural Probes. Plos ONE. 2011 doi: 10.1371/journal.pone.0026204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heo C., Park H., Kim Y.-T., Baeg E., Kim Y.H., Kim S.-G., Minah S. A soft, transparent, freely accessible cranial window for chronic imaging and electrophysiology. Sci. Rep. 2016;6(1):1–11. doi: 10.1038/srep27818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes I.E. Do computer simulations of laboratory practicals meet learning needs? Trends Pharmacol. Sci. 2001;22(2):71–74. doi: 10.1016/s0165-6147(00)01605-9. [DOI] [PubMed] [Google Scholar]
- Jun J.J., Steinmetz N.A., Siegle J.H., Denman D.J., Bauza M., Barbarits B., Lee A.K., Anastassiou C.A., Andrei A., Aydın Ç., Barbic M., Blanche T.J., Bonin V., Couto J., Dutta B., Gratiy S.L., Gutnisky D.A., Häusser M., Karsh B., Ledochowitsch P., Lopez C.M., Mitelut C., Musa S., Okun M., Pachitariu M., Putzeys J., Rich P.D., Rossant C., Sun W., Svoboda K., Carandini M., Harris K.D., Koch C., O’Keefe J., Harris T.D. Fully integrated silicon probes for high-density recording of neural activity. Nature. 2017 doi: 10.1038/nature24636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk R.G.W. Recovering the principles of humane experimental technique. Sci. Technol. Human Values. 2018;43(4):622–648. doi: 10.1177/0162243917726579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanzanova M., Mucchetti G., Neviani E. Analysis of conductance changes as a growth index of lactic acid bacteria in milk. J. Dairy Sci. 1993;76(1):20–28. doi: 10.3168/jds.S0022-0302(93)77319-1. [DOI] [PubMed] [Google Scholar]
- Pogue B.W., Patterson M.S. Review of tissue simulating phantoms for optical spectroscopy, imaging and dosimetry. J. Biomed. Opt. 2006;11(4) doi: 10.1117/1.2335429. [DOI] [PubMed] [Google Scholar]
- Pohl B.M., Gasca F., Christ O., Hofmann U.G. 3D printers May reduce animal numbers to train neuroengineering procedures. 2013 6th International IEEE/EMBS Conference on Neural Engineering (NER. 2013:887–890. [Google Scholar]
- Russell W.M.S., Burch R.L. Methuen & Co. Ltd.; London: 1959. The Principles of Humane Experimental Technique. [Google Scholar]
- Schmidt A., Hohensee C., Teichgräber U., Schmidt A. SATIS ethics ranking of universities in Germany regarding animal use in education. ALTEX. 2011;28(3):243–244. [PubMed] [Google Scholar]
- Stevens C.A., Dey N.D. A program for simulated rodent surgical training. Lab Anim. 2007;36(9):25–31. doi: 10.1038/laban1007-25. [DOI] [PubMed] [Google Scholar]
- Tannenbaum J., Taylor Bennett B. Russell and Burch’s 3Rs then and now: the need for clarity in definition and purpose. J. Am. Assoc. Lab. Anim. Sci. 2015;54(2):120–132. [PMC free article] [PubMed] [Google Scholar]