Abstract
Icariin, a flavonoid isolated from traditional oriental herbal medicines, has been demonstrated to exhibit several health benefits in animal models and in humans. The aim of the present study was to investigate the effect of Icariin on hyperglycemia in type 2 diabetes mellitus (T2DM) in rats. A model of diabetes was established in 50 Sprague Dawley rats using a high-sugar and high-fat diet and peritoneal injection of streptozotocin. Diabetic rats were divided into five groups: Diabetic control; metformin; and rats treated with three different doses of Icariin, 5, 10 and 20 mg/kg. Body weight and blood glucose levels were measured, and serum adiponectin levels, expression of phospho-AMP mediated protein kinase (p-AMPK) and glucose transporter isoform 4 (GLUT-4) were measured using ELISA, Realtime PCR and western blotting, respectively. Diabetic rats without drug treatment exhibited reduced body weight, increased blood glucose levels and decreased the number of islets. In T2DM rats treated with 10 or 20 mg/kg Icariin, the blood glucose levels were reduced, whereas serum adiponectin levels were not affected. Additionally, the mRNA and protein expression levels of p-AMPK and GLUT-4 protein were increased in the T2DM rats treated with Icariin. In conclusion, in the diabetes rat model, Icariin alleviated the severity of diabetes, and the effects may be associated with reduction of hyperglycemia by activating an AMPK/GLUT-4 pathway.
Keywords: Icariin, type 2 diabetes mellitus, AMP-mediated protein kinase, glucose transporter isoform 4
Introduction
Diabetes is a worldwide health problem with a prevalence of ~6% in adults (1). Type 2 diabetes mellitus (T2DM) accounts for 90-95% of all diabetic cases and is characterized by insulin resistance and impaired glucose and lipid metabolism (2). Treatment of diabetes and its complications are primarily dependent on chemical and biological agents, which are associated with certain side effects, including gastrointestinal problems and hypoglycemia. Natural medicines have exhibited anti-diabetic activity (3,4). Icariin (C30H40O15; molecular weight, 676.67), the molecular structure of which is shown in Fig. 1(5), is a flavonoid isolated from the traditional oriental herbal medicine, Epimedium koreanum Nakai. Icariin exhibits a variety of beneficial biological activities, including immunological functions (6), sexual function (7), cardiovascular diseases (8), and anti-cancer (9) and anti-Alzheimer's disease effects (10). In rats, Icariin was also found to alleviate renal damage (11), enhance neurite growth in retinal ganglion cells (12), ameliorate signs of impotence (13) and lower lipid levels (14). However, there is no direct evidence demonstrating how Icariin regulates glucose homeostasis.
Adiponectin is a biologically active polypeptide produced by adipocytes (15). Adiponectin shows anti-diabetic potential by improving insulin sensitivity (16,17). AMP-mediated protein kinase (AMPK) is a key molecule involved in regulation of energy metabolism, by increasing the ratio of intracellular AMP/ATP (18-20). Additionally, LKB1, an upstream kinase of the AMPK pathway, activates AMPK, promoting the phosphorylation of Thr172. Accordingly, LKB1, regulates glucose absorption during contractions of muscles (21). Drugs which regulate adiponectin levels or the AMPK-mediated pathway exhibit hyperglycemic actions which may be used for the treatment of diabetes (22,23).
Defects in skeletal muscle function have been associated with insulin resistance in diabetes (24). Glucose transporter isoform 4 (GLUT-4) expression is upregulated in skeletal muscle and adipose tissues (25). Insulin promotes intracellular GLUT-4 translocation to the cytoplasmic membrane, increasing glucose uptake in skeletal muscle (26). Exercise increases GLUT-4 expression and AMPK activation in skeletal muscles (27,28). Overexpression of GLUT-4 improves glucose homeostasis (29). Flavonoids function as an antidiabetic, primarily by increasing the expression of and promoting translocation of GLUT-4 via the AMPK signaling pathway (4). The results of the present study suggest that regulation of the AMPK/GLUT-4 pathway in skeletal muscles may be an effective potential therapy for treatment of hyperglycemia.
The primary aim of the present study was to investigate the effects of Icariin on the levels of glucose in a rat model of diabetes. Additionally, the role of AMPK/GLUT-4 signaling pathway in the antidiabetic effects of Icariin were examined.
Materials and methods
Animal models
Animal experiments were performed in accordance with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (publication no. 85-23, revised 1996). The present study was approved by the Animal Ethics Committee of Qingdao University. Sixty five-week-old male Sprague-Dawley rats, (100-120 g) provided by the Institute of Qingdao Platford Breeding Co., were maintained in a pathogen-free environment with a 12 h light/dark cycle with free access to food and water. The diabetic group (n=50) was fed with high-sugar and high-fat diet (kcal%: 45% fat, 20% protein, and 35% 100 carbohydrate; 4.73 kcal/gm, Research Diet, New Brunswick, NJ, USA) for 4 weeks (30), whereas the control group was fed with a normal diet for 4 weeks. Diabetes was induced by intraperitoneal injection of 40 mg/kg streptozotocin (STZ; S0130, Sigma).
Three days after STZ injection, T2DM was confirmed, as blood glucose levels were increased. A total of 50 rats with diabetes were randomly divided into five groups (n=10 per group): Diabetic control; metformin (400 mg/kg dissolved in water, administered by gavage) (31); and rats treated with either 5, 10 or 20 mg/kg Icariin (32)(489-32-7, Sigma) dissolved in carboxymethylcellulose sodium administered by intraperitoneal injection, once a day. 10 normal rats served as the control group. After a total of 3 weeks of drugs treatment, the body weight and fasting blood glucose levels were recorded. All the experimental animals survived.
Blood sample collection and tissue extraction
First of all, rats were anesthetized with 30 mg/kg sodium pentobarbital. Then, blood samples were collected from tail veins. An oral glucose tolerance test, in which 20% glucose was fed with a syringe at a dose of 2 g/kg, was performed after the rats were fasted for 10 h (33). Blood samples were collected from the caudal vein by means of a small incision at the end of the tail at 0, 15, 30, 60 and 120 min after the glucose administration. Subsequently, the level of blood glucose was measured.
After OGTT test, rats were euthanized using 150 mg/kg sodium pentobarbital. Pancreatic tissues were dissected, processed as paraffin blocks, then stained with hematoxylin eosin. Pancreatic tissues were rehydrated, incubated, washed, rapidly dehydrated and subsequently mounted on cover slips. Tissues were imaged using a microscope (DM750M, Leica) at x200 magnification.
Serum adiponectin measurement
Serum adiponectin concentrations were determined using a specific ELISA kit (ab108786, Abcam).
RNA extraction and gene microarray hybridization
Total RNA was extracted from bisected soleus muscle tissue using an RNA isolation kit (AM1912, Invitrogen, America). RNA concentrations were measured using spectrophotometric analysis by measuring the A260/280 ratio. The instrument for detecting RNA concentration is spectrophotometer (E300, Thermo, America). Reverse transcription-quantitative PCR was performed and analyzed on a Rotor-Gene 6000 system (Corbett Research). PCR was performed using a SYBR® Premix Ex Taq™ (Tli RNaseH Plus) kit (RR420A, Takara). The thermocycling conditions were: Initial denaturation, 9˚C for 30 sec; followed by 40 cycles of denaturation at 60˚C for 30 sec, primer annealing at 9˚C for 5 sec, and extension at 64˚C for 1 min. Fluorescence was measured at 72˚C in each cycle. To determine the specificity of PCR reactions, melt curve analysis was performed following amplification by slowly ramping the heat from 72˚C to 9˚C, with fluorescence acquisition at 1˚C intervals and a 5-sec hold at each increment. The forward and reverse primer sequences were as follows: GLUT-4 forward, 5'-TCATTCCTGTGAAAGTGATGACGA-3' and reverse, 5'-CTGCCACAGTGTCATATCATCCAA-3'; and β-actin forward, 5'-CCGTAAAGACCTCTATGCCAACA-3' and reverse 5'-GCTAGGAGCCAGGGCAGTAATC-3'. Expression of the target gene was normalized to β-actin. Primer 5 software was used for the primer design.
Western blotting
Homogenized skeletal muscle (0.1 g) at 4˚C in 1 ml of lysis buffer containing 50 mM Tris.HCl, pH 7.4, 150 mM NaCl, 1 mM EDTA, 1% Nonidet P-40, 5 mM Na3VO4, 20 mM NaF, 10 mM sodium pyrophosphate and 50 µl protease inhibitor cocktail (B14001, Bimake). Equal quantities (50 µg) of total protein were resolved on a 12% gel using SDS-PAGE and transferred to a PVDF membrane (FFP32, Beyotime). After transfer, membranes were blocked using 5% skimmed milk containing 0.1% Tween-20. Subsequently the membranes were incubated with anti-GLUT-4 (2213S, Cell Signaling Technology, America), anti-phospho (p)-AMPK (2535S, Cell Signaling Technology), total-AMPK (2532S, Cell Signaling Technology) or anti-β-actin (ab8227, Abcam). Membranes were then incubated with horse radish peroxidase (HRP)-conjugated secondary antibodies, including HRP-conjugated goat anti-rabbit immunoglobulin G (IgG) (AP132P, Merck Millipore), or HRP-conjugated goat anti-mouse IgG (ab6789, Abcam). Enhanced chemiluminescence plus kit (PE0010, Solarbio) was used to visualize the signals. Relative protein expression levels were quantified using densitometry analysis and normalized to β-actin expression levels.
Statistical analysis
GraphPad Prism version 4.0 (GraphPad Software, Inc.) was used to analyze the data. Statistical analysis was performed using a one-way ANOVA with a Tukey's post hoc test. All data are presented as the mean ± standard deviation. P<0.05 was considered to indicate a statistically significant difference.
Results
The effect of Icariin on body weights, blood glucose levels and serum fasting blood glucose levels in diabetic rats
After 3 weeks of treatment with drugs, the body weight of rats decreased significantly (*P<0.05; Table I) compared with the non-diabetic control. There was no significant difference in the body weight of rats treated with 5 mg/kg Icariin compared with the diabetic control (P>0.05; Table I). However, the body weights of rats were significantly increased when treated with 10 or 20 mg/kg Icariin compared with the diabetic control (^P<0.05, Table I). There was no significant difference in body weight between the Icariin (10 and 20 mg/kg) group and the metformin group (P>0.05, vs. the metformin group, Table I).
Table I.
The effect of Icariin on body weight in T2DM rats.
| Groups | Basal body weight (g) | Body weight (g) 21 days after drug treatment |
|---|---|---|
| Control | 201.8±9.8 | 223.8±12.1 |
| Diabetic | 198.8±11.4 | 187.8±11.4a |
| Metformin | 201.4±7.2 | 219.3±9.1b |
| Icariin 5 mg/kg | 203.3±10.9 | 195.3±9.7 |
| Icariin 10 mg/kg | 202.3±11.2 | 208.7±6.9b |
| Icariin 20 mg/kg | 200.9±8.2 | 215.3±12.1b |
Values are means ± standard deviation.
aP<0.05 vs. the control group;
bP<0.05 vs. the diabetic group; n=10; T2DM, type 2 diabetes mellitus.
The diabetic rats treated with 10 or 20 mg/kg Icariin exhibited reduced blood glucose levels compared with the diabetic control rats (^P<0.05; Table II). Meanwhile, there was no significant difference in blood glucose level between the Icariin (10 and 20 mg/kg) group and the metformin group (P>0.05, vs. the metformin group, Table II). However, the change in blood glucose levels were not considered significant in the rats treated with 5 mg/kg Icariin compared with the diabetic control rats (P>0.05, Table II).
Table II.
The effect of Icariin on blood glucose in T2DM rats.
| Groups | Blood glucose (mmol/l) before STZ injection | Blood glucose (mmol/l) 21 days after drug treatment |
|---|---|---|
| Control | 2.21±0.4 | 2.88±0.7 |
| Diabetic | 2.29±0.5 | 5.89±1.1a |
| Metformin | 2.25±0.5 | 3.16±0.9b |
| Icariin 5 mg/kg | 2.25±0.6 | 5.48±1.3 |
| Icariin 10 mg/kg | 2.35±0.4 | 4.02±1.2b |
| Icariin 20 mg/kg | 2.27±0.7 | 3.27±0.7b |
Values are means ± standard deviation.
aP<0.05 vs. the control group;
bP<0.05 vs. the diabetic group; n=10; T2DM, type 2 diabetes mellitus.
In the oral glucose tolerance test, the blood glucose levels reached peak levels after 30 min, and returned to resting levels after ~120 min, except in rats treated with 5 mg/kg Icariin, where peak blood glucose levels were reached after 60 min. Glucose levels were significantly lower in the rats treated with 10 or 20 mg/kg Icariin compared with the diabetic control (^P<0.05; Table III). There was no significant difference in oral glucose tolerance between the Icariin (10 and 20 mg/kg) group and the metformin group (P>0.05, vs. the metformin group, Table III).
Table III.
The effect of Icariin on blood glucose level during the oral glucose tolerance test in T2DM rats.
| Blood glucose (mmol/l) | ||||||
|---|---|---|---|---|---|---|
| Groups | 0 min | 15 min | 30 min | 60 min | 120 min | |
| Control | 2.88±0.7 | 6.45±1.3 | 7.09±1.2 | 6.24±1.0 | 3.87±0.7 | |
| Diabetic | 5.89±1.1 | 12.3±1.8 | 19.3±1.0 | 20.51±1.1 | 3.83±1.8a | |
| Metformin | 3.16±0.9 | 8.23±1.7 | 9.63±1.4 | 7.98±1.3 | 3.82±1.1b | |
| Icariin 5 mg/kg | 5.48±1.3 | 8.70±1.3 | 12.88±1.8 | 13.86±2.1 | 9.83±2.3 | |
| Icariin 10 mg/kg | 4.02±1.2 | 10.31±1.9 | 11.41±0.9 | 10.87±1.5 | 5.31±1.3b | |
| Icariin 20 mg/kg | 3.27±0.7 | 8.29±1.7 | 9.81±1.6 | 8.68±1.8 | 4.03±1.8b | |
Values are means ± standard deviation.
aP<0.05 vs. the control group;
bP<0.05 vs. the diabetic group; n=10; T2DM, type 2 diabetes mellitus.
The effect of Icariin on pancreatic tissues in diabetic rats
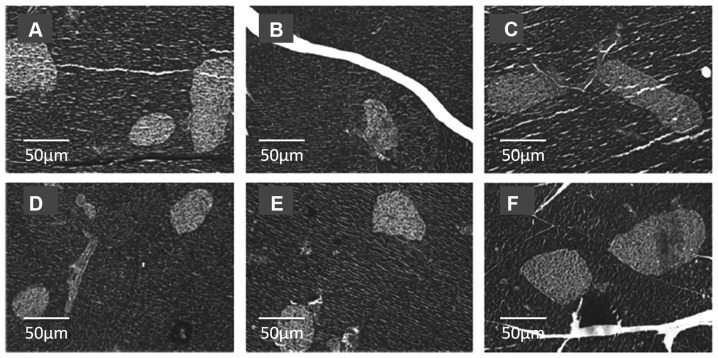
The morphology of islets in the pancreatic tissues from the different groups are shown in Fig. 2. STZ treatment resulted in impaired pancreatic tissues, with fewer islets compared with the normal control (Fig. 2A and B, respectively). Icariin treatment reduced the loss in the number of islets, compared with the diabetic control rats, irrespective of the dose used, thus serving a protective role during the diabetic process (Fig. 2D-F). Metformin treatment also reduced the loss in the number of islets, compared with the diabetic control rats (Fig. 2C).
Figure 2.
Images of pancreatic tissues stained with hematoxylin and eosin. Islets are colored lighter compared with the rest of the pancreatic tissue. Representative images of pancreatic tissues from (A) normal control, (B) diabetic control, (C) metformin control, and type 2 diabetes mellitus rats treated with (D) 5, (E) 10 or (F) 20 mg/kg Icariin. Magnification, x200.
The effect of Icariin on serum adiponectin levels in diabetic rats
STZ treatment increased the serum adiponectin levels compared with the non-diabetic control (Fig. 3; *P<0.05); However, no significant changes were observed in the serum adiponectin levels in the rats treated with Icariin (P>0.05, vs. the diabetic group).
Figure 3.
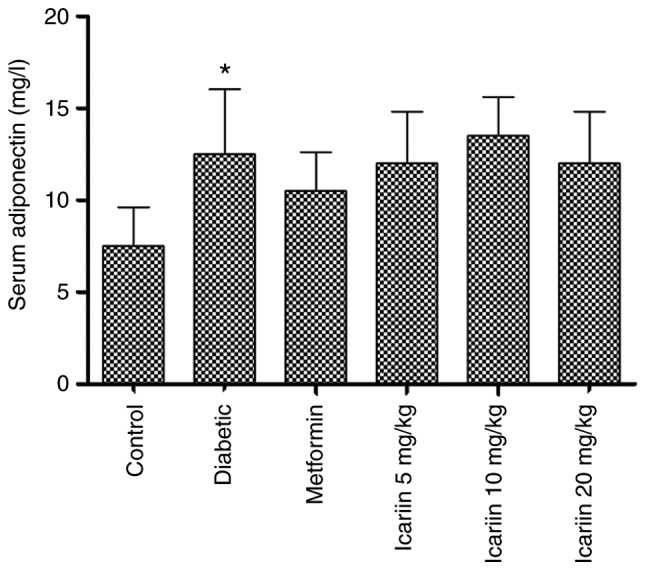
Effect of Icariin on serum adiponectin levels in type 2 diabetes mellitus rats. Data are presented as the mean ± standard deviation. *P<0.05 vs. control group. n=10.
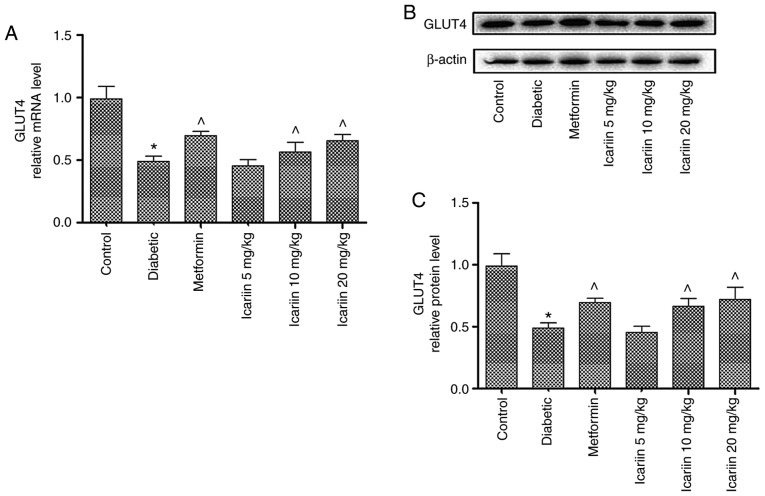
The effect of Icariin on the mRNA and protein expression levels of GLUT-4 in diabetic rats
As shown in Fig. 4, the GLUT-4 mRNA expression levels in skeletal muscles were significantly lower in the diabetic rats compared with the control (P<0.05). In the rats treated with 10 and 20 mg/kg Icariin, GLUT-4 mRNA expression levels were significantly increased compared with the diabetic control (Fig. 4A; ^P<0.05). Similarly, GLUT-4 protein expression levels were also increased in Icariin treated mice compared with the diabetic control (Fig. 4B and C; P<0.05). There was no significant difference in expression of GLUT-4 between the Icariin (10 and 20 mg/kg) group and the metformin group (P>0.05, vs. the metformin group).
Figure 4.
Effect of Icariin on the mRNA and protein expression levels of GLUT-4 in diabetic rats. (A) mRNA and (B) protein expression levels of GLUT-4 in type 2 diabetes mellitus rats in the normal control, diabetic control, metformin control, and type 2 diabetes mellitus rats treated with 5, 10 or 20 mg/kg Icariin. (C) Statistical analysis. Data were presented as the ratio of GLUT-4 to β-actin. Data are presented as the mean ± standard deviation. n=10 per group. ^P<0.05 vs. diabetic group. *P<0.05 vs. control group. GLUT-4, glucose transporter isoform 4.
The effect of Icariin on phosphorylation of AMPK in diabetic rats
The phosphorylation of AMPK was decreased in the diabetic rats compared with the control (*P<0.05; Fig. 5). Phosphorylation of AMPK in the rats treated with 10 or 20 mg/kg Icariin was significantly increased compared with the diabetic control (P<0.05; Fig. 5). There was no significant difference in phosphorylation of AMPK between the Icariin (10 and 20 mg/kg) group and the metformin group (P>0.05, vs. the metformin group, Fig. 5).
Figure 5.
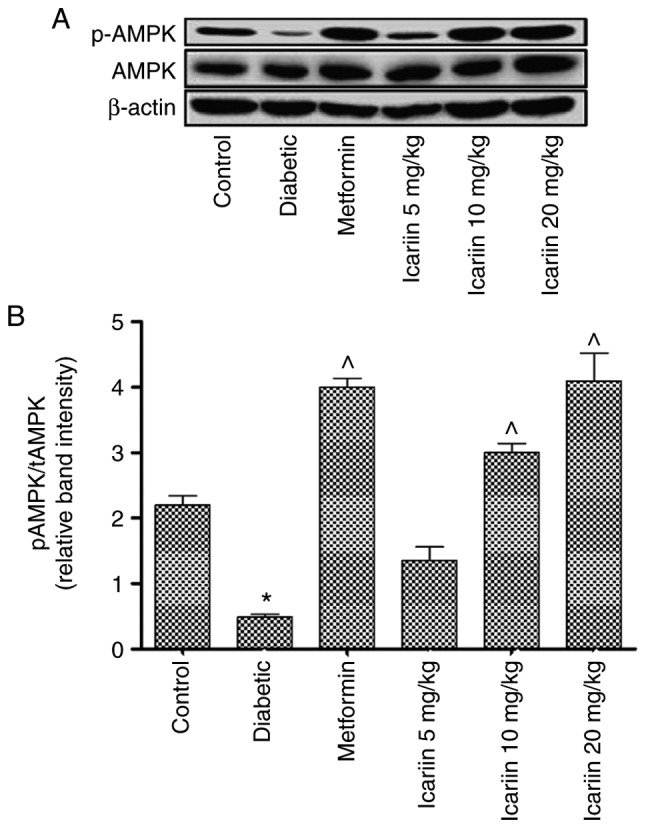
Effect of Icariin on phosphorylation of AMPK in diabetic rats. (A) Protein expression levels of phosphorylated of AMPK in type 2 diabetes mellitus rats in the normal control, diabetic control, metformin control, and type 2 diabetes mellitus rats treated with 5, 10 or 20 mg/kg Icariin. (B) Statistical analysis. Data were presented as the ratio of phosphorylated AMPK to total AMPK. Data are presented as the mean ± standard deviation. n=10 per group. *P<0.05 vs. control group; ^P<0.05 vs. the diabetic group.
Discussion
The primary findings of the present study were that treatment with 10 or 20 mg/kg Icariin for 3 weeks reduced the blood glucose levels in diabetic rats. This treatment also reduced the peak glucose levels in an oral glucose tolerance test. Furthermore, treatment with Icariin resulted in reducing the loss in the number of islets in the pancreatic tissues and treatment with Icariin was associated with upregulated mRNA expression of GLUT-4 and increased phosphorylation of AMPK in the skeletal muscles. These results suggest that the beneficial effects of Icariin on T2DM may be associated with an AMPK/GLUT-4 signaling pathway.
Recent studies have suggested that polyphenolic compounds prevent the development of long-term diabetes and its complications, including cardiovascular disease, neuropathy, nephropathy and retinopathy (34,35). The therapeutic properties of Epimedium koreanum have been attributed to the flavonoid component of Icariin, which has been reported to exhibit a broad range of pharmacological effects, including anti-diabetic, anti-Alzheimer's disease, anti-tumor and hepatoprotective properties (36). To the best of our knowledge, the present study is the first to demonstrate the dose-dependent antidiabetic effect of Icariin, with hypoglycemic effects observed with 10 and 20 mg/kg. Treatment with Icariin for 3 weeks reduced the blood glucose levels as well as the peak glucose levels following a bolus dose of glucose.
Metformin is medically considered as the only biguanide which is used and recommended as oral anti-diabetic agent, which is crucial for decreasing the levels of plasma glucose. As known, metformin has been found to exert an increasing effect on inhibiting hepatic gluconeogenesis, decreasing hyperinsulinemia, reducing protein synthesis, improving insulin sensitivity and enhancing glucose use in the muscle. In clinical practice, previous evidence has reported that metformin is widely accepted as an effective treatment for DM, and notably to T2DM by serving as the first-line therapy. Therefore, metformin was chosen as a positive control drug in this study.
Adipose tissue has been demonstrated to serve an endocrine role in recent years. Adiponectin, secreted by adipocytes, is an insulin-sensitizing hormone (15), improving insulin resistance in mice (37). And in studies on humans, adiponectin has the potential to be a biomarker for predicting metabolic diseases such as diabetes mellitus (38). In clinical trials, adiponectin was demonstrated to exhibit anti-diabetic (39), anti-atherosclerotic (40) and anti-cancer potential (41). Adiponectin improves insulin sensitivity by increasing insulin receptor expression and signal transduction, thereby alleviating insulin resistance (16,17,42). In the present study, there was no statistically significant difference in the adiponectin levels between the Icariin treated and diabetes control group, suggesting that the anti-diabetes effect of Icariin was likely not associated with the expression of adiponectin in the rat model diabetes used.
AMPK, a crucial component of cellular metabolism, has been demonstrated to inhibit many metabolic diseases including T2DM. Metformin lowers blood glucose levels by inhibiting hepatic glucose production, which is mediated by an AMPK-dependent mechanism (43). Increased glucose uptake following AMPK activation by AICA-riboside in perfused rat hindlimb muscles is attributed to an increase in translocation of GLUT-4 to the cell membrane (44). These results suggest that increasing GLUT-4 expression in skeletal muscles may be an effective therapy for treating diabetes. In the present study, Icariin treatment resulted in increased expression of AMPK and GLUT-4, suggesting that the anti-hyperglycemic effects of Icariin may be associated with the AMPK/GLUT-4 signaling pathway.
The limitation of this study is that there is no measurement of insulin levels under Icariin intervention. Thus, it is impossible to accurately assess the islet function. This study does not investigate whether Icariin has side effects in the treatment of T2DM. In this study, we did not observe the effect of Icariin on healthy rats. The side effects of Icariin were not elucidated by literature search. However, Icariin belongs to flavonoids which have common side effects (45), such as allergic reaction and pyrogen reaction. Studies have shown that the toxic side effects may be caused by the charge complexes formed by a class of lipoproteins and flavonoids. To clarify the common impurities and physicochemical properties of these flavonoids, effective separation methods should be adopted to provide safe and effective drugs for clinical use. Finally, there was no data on diabetic patients. This is also what needs to be done in future research.
In summary, the present study demonstrated that Icariin is an effective therapy for treating diabetes in a rat model of T2DM. The pharmacological effects of Icariin is related to preserve pancreatic islet number or function and increased expression levels of AMPK and GLUT-4 in the skeletal muscles.
Figure 1.
Chemical structure of Icariin.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the National Natural Science Foundation of China (grant no. 81601103) and the China Postdoctoral Science Foundation (grant no. 2016M602100).
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
YC and LW made substantial contributions to conception, design of the experiments, as well as the writing and revision of this manuscript. XL was responsible for acquisition and interpretation of data. XL, YW, PS, YL, TL and SL performed the experiments and participated in the writing of the manuscript. CW analyzed the data. The manuscript has been read and approved by each author, and all agree to this submission.
Ethics approval and consent to participate
The current study was approved by the Medical Ethics Committee of Affiliated Hospital of Qingdao University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Ogurtsova K, da Rocha Fernandes JD, Huang Y, Linnenkamp U, Guariguata L, Cho NH, Cavan D, Shaw JE, Makaroff LE. IDF Diabetes Atlas: Global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract. 2017;128:40–50. doi: 10.1016/j.diabres.2017.03.024. [DOI] [PubMed] [Google Scholar]
- 2.Constantin RP, Constantin RP, Bracht A, Yamamoto NS, Ishii-Iwamoto EL, Constantin J. Molecular mechanisms of citrus flavanones on hepatic gluconeogenesis. Fitoterapia. 2014;92:148–162. doi: 10.1016/j.fitote.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 3.Li WL, Zheng HC, Bukuru J, De Kimpe N. Natural medicines used in the traditional Chinese medical system for therapy of diabetes mellitus. J Ethnopharmacol. 2004;92:1–21. doi: 10.1016/j.jep.2003.12.031. [DOI] [PubMed] [Google Scholar]
- 4.Hajiaghaalipour F, Khalilpourfarshbafi M, Arya A. Modulation of glucose transporter protein by dietary flavonoids in type 2 diabetes mellitus. Int J Biol Sci. 2015;11:508–524. doi: 10.7150/ijbs.11241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xiong W, Ma X, Wu Y, Chen Y, Zeng L, Liu J, Sun W, Wang D, Hu Y. Determine the structure of phosphorylated modification of icariin and its antiviral activity against duck hepatitis virus A. BMC Vet Res. 2015;11(205) doi: 10.1186/s12917-015-0459-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.He W, Sun H, Yang B, Zhang D, Kabelitz D. Immunoregulatory effects of the herba Epimediia glycoside icariin. Arzneimittelforschung. 1995;45:910–913. [PubMed] [Google Scholar]
- 7.Makarova MN, Pozharitskaya ON, Shikov AN, Tesakova SV, Makarov VG, Tikhonov VP. Effect of lipid-based suspension of Epimedium koreanum Nakai extract on sexual behavior in rats. J Ethnopharmacol. 2007;114:412–416. doi: 10.1016/j.jep.2007.08.021. [DOI] [PubMed] [Google Scholar]
- 8.Xu HB, Huang ZQ. Vasorelaxant effects of icariin on isolated canine coronary artery. J Cardiovasc Pharmacol. 2007;49:207–213. doi: 10.1097/FJC.0b013e3180325abe. [DOI] [PubMed] [Google Scholar]
- 9.Zhou J, Wu J, Chen X, Fortenbery N, Eksioglu E, Kodumudi KN, Pk EB, Dong J, Djeu JY, Wei S. Icariin and its derivative, ICT, exert anti-inflammatory, anti-tumor effects, and modulate myeloid derived suppressive cells (MDSCs) functions. Int Immunopharmacol. 2011;11:890–898. doi: 10.1016/j.intimp.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen YJ, Zheng HY, Huang XX, Han SX, Zhang DS, Ni JZ, He XY. Neuroprotective effects of icariin on brain metabolism, mitochondrial functions, and cognition in triple-transgenic alzheimer's disease mice. CNS Neurosci Ther. 2016;22:63–73. doi: 10.1111/cns.12473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qi MY, Kai-Chen Liu HR, Su YH, Yu SQ. Protective effect of Icariin on the early stage of experimental diabetic nephropathy induced by streptozotocin via modulating transforming growth factor beta1 and type IV collagen expression in rats. J Ethnopharmacol. 2011;138:731–736. doi: 10.1016/j.jep.2011.10.015. [DOI] [PubMed] [Google Scholar]
- 12.Xin H, Zhou F, Liu T, Li GY, Liu J, Gao ZZ, Bai GY, Lu H, Xin ZC. Icariin ameliorates streptozotocin-induced diabetic retinopathy in vitro and in vivo. Int J Mol Sci. 2012;13:866–878. doi: 10.3390/ijms13010866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang ZB, Yang QT. The testosterone mimetic properties of icariin. Asian J Androl. 2006;8:601–605. doi: 10.1111/j.1745-7262.2006.00197.x. [DOI] [PubMed] [Google Scholar]
- 14.Lu YF, Xu YY, Jin F, Wu Q, Shi JS, Liu J. Icariin is a PPARα activator inducing lipid metabolic gene expression in mice. Molecules. 2014;19:18179–18191. doi: 10.3390/molecules191118179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hossain MM, Mukheem A, Kamarul T. The prevention and treatment of hypoadiponectinemia-associated human diseases by up-regulation of plasma adiponectin. Life Sci. 2015;135:55–67. doi: 10.1016/j.lfs.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 16.El Husseny MW, Mamdouh M, Shaban S, Ibrahim Abushouk A, Zaki MM, Ahmed OM, Abdel-Daim MM. Adipokines: Potential therapeutic targets for vascular dysfunction in type ii diabetes mellitus and obesity. J Diabetes Res. 2017;2017(8095926) doi: 10.1155/2017/8095926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao Q, Yao X, Zheng J. MiR-323 inhibits prostate cancer vascularization through adiponectin receptor. Cell Physiol Biochem. 2015;36:1491–1498. doi: 10.1159/000430313. [DOI] [PubMed] [Google Scholar]
- 18.Ye JM, Dzamko N, Hoy AJ, Iglesias MA, Kemp B, Kraegen E. Rosiglitazone treatment enhances acute AMP-activated protein kinase-mediated muscle and adipose tissue glucose uptake in high-fat-fed rats. Diabetes. 2006;55:2797–2804. doi: 10.2337/db05-1315. [DOI] [PubMed] [Google Scholar]
- 19.Fujii N, Ho RC, Manabe Y, Jessen N, Toyoda T, Holland WL, Summers SA, Hirshman MF, Goodyear LJ. Ablation of AMP-activated protein kinase alpha2 activity exacerbates insulin resistance induced by high-fat feeding of mice. Diabetes. 2008;57:2958–2966. doi: 10.2337/db07-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Richter EA, Ruderman NB. AMPK and the biochemistry of exercise: Implications for human health and disease. Biochem J. 2009;418:261–275. doi: 10.1042/BJ20082055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sakamoto K, McCarthy A, Smith D, Green KA, Grahame Hardie D, Ashworth A, Alessi DR. Deficiency of LKB1 in skeletal muscle prevents AMPK activation and glucose uptake during contraction. EMBO J. 2005;24:1810–1820. doi: 10.1038/sj.emboj.7600667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sharma AX, Quittner-Strom EB, Lee Y, Johnson JA, Martin SA, Yu X, Li J, Lu J, Cai Z, Chen S, et al. Glucagon receptor antagonism improves glucose metabolism and cardiac function by promoting AMP-mediated protein kinase in diabetic mice. Cell Rep. 2018;22:1760–1773. doi: 10.1016/j.celrep.2018.01.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Na RS, Ma C, Liu QR, Wu LM, Zheng XL, Liu ZW. Itraconazole attenuates hepatic gluconeogenesis and promotes glucose uptake by regulating AMPK pathway. Exp Ther Med. 2018;15:2165–2171. doi: 10.3892/etm.2017.5602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lowell BB, Shulman GI. Mitochondrial dysfunction and type 2 diabetes. Science. 2005;307:384–387. doi: 10.1126/science.1104343. [DOI] [PubMed] [Google Scholar]
- 25.James DE, Strube M, Mueckler M. Molecular cloning and characterization of an insulin-regulatable glucose transporter. Nature. 1989;338:83–87. doi: 10.1038/338083a0. [DOI] [PubMed] [Google Scholar]
- 26.Goodyear LJ, Kahn BB. Exercise, glucose transport, and insulin sensitivity. Annu Rev Med. 1998;49:235–261. doi: 10.1146/annurev.med.49.1.235. [DOI] [PubMed] [Google Scholar]
- 27.Kraniou Y, Cameron-Smith D, Misso M, Collier G, Hargreaves M. Effects of exercise on GLUT-4 and glycogenin gene expression in human skeletal muscle. J Appl Physiol (1985) 2000;88:794–796. doi: 10.1152/jappl.2000.88.2.794. [DOI] [PubMed] [Google Scholar]
- 28.Cao S, Li B, Yi X, Chang B, Zhu B, Lian Z, Zhang Z, Zhao G, Liu H, Zhang H. Effects of exercise on AMPK signaling and downstream components to PI3K in rat with type 2 diabetes. PLoS One. 2012;7(e51709) doi: 10.1371/journal.pone.0051709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leturque A, Loizeau M, Vaulont S, Salminen M, Girard J. Improvement of insulin action in diabetic transgenic mice selectively overexpressing GLUT4 in skeletal muscle. Diabetes. 1996;45:23–27. doi: 10.2337/diab.45.1.23. [DOI] [PubMed] [Google Scholar]
- 30.Zhang Q, Xiao X, Zheng J, Li M, Yu M, Ping F, Wang T, Wang X. doi: 10.1042/BSR20180059. Liraglutide protects cardiac function in diabetic rats through the PPAα pathway. Biosci Rep, 2018 (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ismail TA, Soliman MM, Nassan MA. Molecular and immunohistochemical effects of metformin in a rat model of type 2 diabetes mellitus. Exp Ther Med. 2015;9:1921–1930. doi: 10.3892/etm.2015.2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang X, Liu C, Xu Y, Chen P, Shen Y, Xu Y, Zhao Y, Chen W, Zhang X, Ouyang Y, et al. Combination of mesenchymal stem cell injection with icariin for the treatment of diabetes-associated erectile dysfunction. PLoS One. 2017;12(e0174145) doi: 10.1371/journal.pone.0174145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Du XX, Tao X, Liang S, Che JY, Yang S, Li H, Chen JG, Wang CM. Hypoglycemic effect of acidic polysaccharide from schisandra chinensis on T2D rats induced by high-fat diet combined with STZ. Biol Pharm Bull. 2019;42:1275–1281. doi: 10.1248/bpb.b18-00915. [DOI] [PubMed] [Google Scholar]
- 34.Bahadoran Z, Mirmiran P, Azizi F. Dietary polyphenols as potential nutraceuticals in management of diabetes: A review. J Diabetes Metab Disord. 2013;12(43) doi: 10.1186/2251-6581-12-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang Y, Zhen W, Maechler P, Liu D. Small molecule kaempferol modulates PDX-1 protein expression and subsequently promotes pancreatic β-cell survival and function via CREB. J Nutr Biochem. 2013;24:638–646. doi: 10.1016/j.jnutbio.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim DH, Jung HA, Sohn HS, Kim JW, Choi JS. Potential of Icariin metabolites from Epimedium koreanum Nakai as antidiabetic therapeutic agents. Molecules. 2017;22 doi: 10.3390/molecules22060986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, Mori Y, Ide T, Murakami K, Tsuboyama-Kasaoka N, et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med. 2001;7:941–946. doi: 10.1038/90984. [DOI] [PubMed] [Google Scholar]
- 38.Nie JM, Li HF. Metformin in combination with rosiglitazone contribute to the increased serum adiponectin levels in people with type 2 diabetes mellitus. Exp Ther Med. 2017;14:2521–2526. doi: 10.3892/etm.2017.4823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Adibian M, Hodaei H, Nikpayam O, Sohrab G, Hekmatdoost A, Hedayati M. The effects of curcumin supplementation on high-sensitivity C-reactive protein, serum adiponectin, and lipid profile in patients with type 2 diabetes: A randomized, double-blind, placebo-controlled trial. Phytother Res. 2019;33:1374–1383. doi: 10.1002/ptr.6328. [DOI] [PubMed] [Google Scholar]
- 40.Nakajima T, Yokota T, Shingu Y, Yamada A, Iba Y, Ujihira K, Wakasa S, Ooka T, Takada S, Shirakawa R, et al. Impaired mitochondrial oxidative phosphorylation capacity in epicardial adipose tissue is associated with decreased concentration of adiponectin and severity of coronary atherosclerosis. Sci Rep. 2019;9(3535) doi: 10.1038/s41598-019-40419-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tumminia A, Vinciguerra F, Parisi M, Graziano M, Sciacca L, Baratta R, Frittitta L. Adipose tissue, obesity and adiponect in: Role in endocrine cancer risk. Int J Mol Sci. 2019;20(pii: E2863) doi: 10.3390/ijms20122863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zeng F, Shi J, Long Y, Tian H, Li X, Zhao AZ, Li RF, Chen T. Adiponectin and endometrial cancer: A systematic review and meta-analysis. Cell Physiol Biochem. 2015;36:1670–1678. doi: 10.1159/000430327. [DOI] [PubMed] [Google Scholar]
- 43.Duca FA, Côté CD, Rasmussen BA, Zadeh-Tahmasebi M, Rutter GA, Filippi BM, Lam TK. Metformin activates a duodenal Ampk-dependent pathway to lower hepatic glucose production in rats. Nat Med. 2015;21:506–511. doi: 10.1038/nm.3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kurth-Kraczek EJ, Hirshman MF, Goodyear LJ, Winder WW. 5' AMP-activated protein kinase activation causes GLUT4 translocation in skeletal muscle. Diabetes. 1999;48:1667–1671. doi: 10.2337/diabetes.48.8.1667. [DOI] [PubMed] [Google Scholar]
- 45.Farzaei MH, Singh AK, Kumar R, Croley CR, Pandey AK, Coy-Barrera E, Kumar Patra J, Das G, Kerry RG, Annunziata G, et al. Targeting inflammation by flavonoids: Novel therapeutic strategy for metabolic disorders. Int J Mol Sci. 2019;20 doi: 10.3390/ijms20194957. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.