Table 1.
Selected Optimization for Ni/Photoredox DCFa
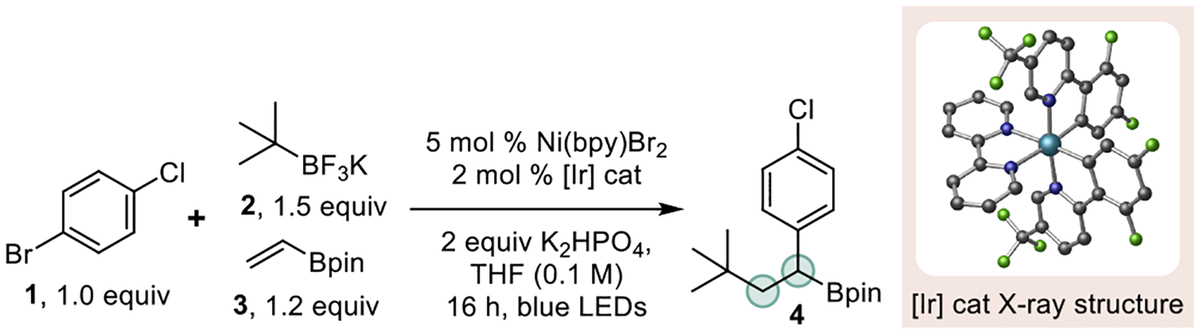
| ||
|---|---|---|
| entry | deviation from standard conditions | NMR yield of 4 |
| 1 | none | 70% (69%)c |
| 2 | no base | 55% |
| 3 | 0.05 M concentration | 67% |
| 4 | 0.25 M concentration | 39% |
| 5 | Kessil lamp in place of blue LEDs | 60% |
| 6 | 5 mol % [Ir] cat, 3 mol % [Ni] | 59% |
| 7 | 5 mol % [Ir] cat, 10 mol % [Ni] | 66% |
| 8 | CI-4CzIPN in place of [Ir] | (40%)c |
| 9 | “zero precautions” | 44%d |
| 10 | No [Ni] catalyst | 0% |
| 11 | No [Ir] photocatalyst | 0% |
| 12 | No light | 0% |
Optimization performed using 4-chloro-1-bromobenzene (0.1 mmol) for 16 h at 27 °C; [Ir] = [Ir{dF(CF3)2ppy}2(bpy)]PF6.
Yields in parentheses are isolated yield of 4 after purification.
Reaction performed using 2,4,5,6-tetrakis(3,6-dichloro-9H-carbazol-9-yl)isophthalonitrile (Cl-4CzIPN) as the photocatalyst.
Reaction performed under air with non-rigorously dried solvent.
