Table 2.
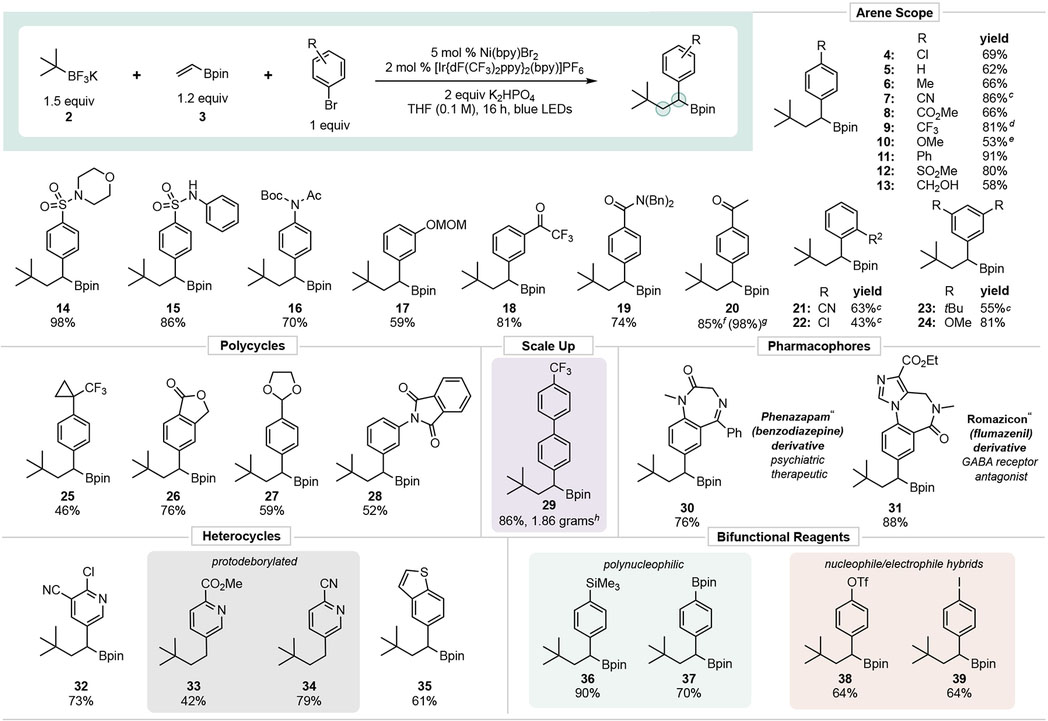
|
Values indicate yield of the isolated product. pin = 2,3-dimethylbutane-2,3-diol.
Conditions unless otherwise noted: aryl halide (1 equiv, 0.5 mmol), 3 (1.2 equiv, 0.6 mmol), 2 (1.5 equiv, 0.75 mmol), [Ir(dFCF3ppy)2bpy]PF6 (2 mol %, 0.010 mmol), Ni(bpy)Br2 (5 mol %, 0.025 mmol), K2HPO4 (2 equiv, 1.0 mmol), THF (0.1 M), 16 h, irradiating with blue LEDs (6 W).
Reaction time extended to 48 h.
Ni(dtbbpy)Br2 used as the Ni catalyst.
Ni(phen)Br2 used as the Ni catalyst.
Isolated yield as BF3K salt.
1H NMR yield of boronate ester
Reaction performed on 5 mmol scale of aryl halide.
