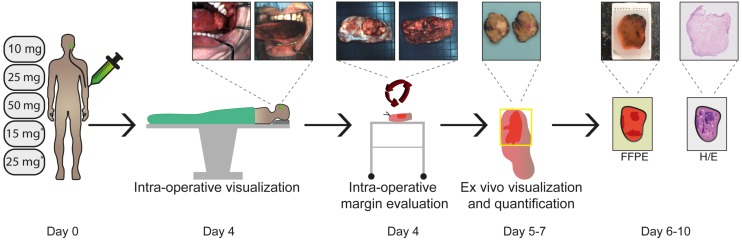
Figure 1.
Study workflow. Five cohorts of three HNC patients received cetuximab-800CW systemically: three single dose cohorts (10, 25, 50 mg) and two cohorts pre-dosed with 75 mg unlabeled cetuximab (15 or 25 mg). Fluorescence visualization was performed before and after excision of the tumor in vivo. Subsequently, back-table fluorescence-guided imaging of the fresh surgical specimen was performed to evaluate the resection margin status. Visualization and quantification of fluorescence was performed during all subsequent steps of standard histopathological processing and correlated to histopathology. Abbreviations: FFPE: Formalin-Fixed Paraffin Embedded, H/E: Hematoxylin and Eosin. *75 mg unlabeled cetuximab is administered one hour prior to cetuximab-800CW administration.