Figure 7.
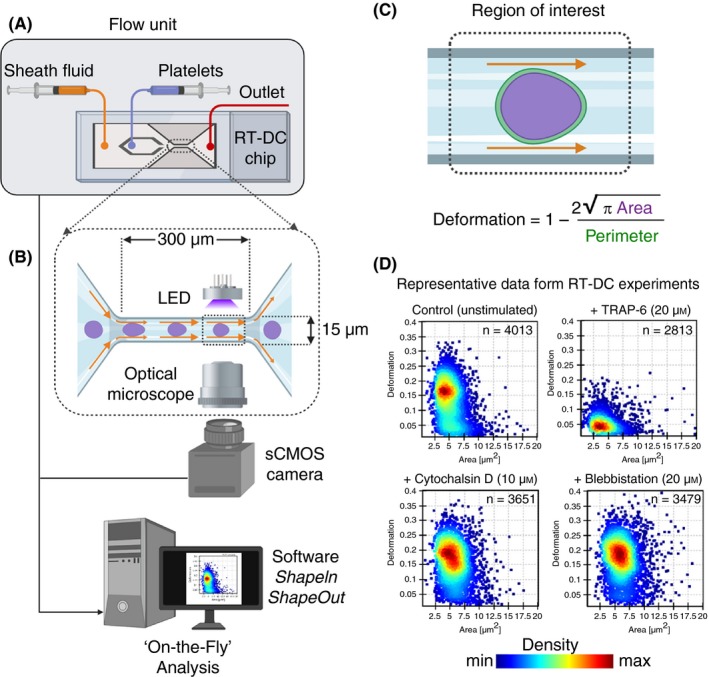
The real‐time deformability cytometry (RT‐DC) microfluidic chip connected to a flow unit (A) consist of dedicated inlets for sheath fluid and for cells/platelets, which combine into to a channel with narrow constriction zone (B) where cell/platelets undergo deformation as a result of hydrodynamic compression brought about by sheath fluid. A high‐powered light‐emitting diode (LED) is used to illuminate the samples. Images are recorded by a scientific complementary metal–oxide–semiconductor (sCMOS) camera working at high frame rates (≈2000 images/s) that is synchronized with the frequency of illumination time. This combined together with microfluidic flow, RT‐DC allows for mechanoprofiling of >100 unique cells/platelets per second in a contact‐ and label‐free manner. Platelet deformation (C) is determined on the fly in real time by computational image processing algorithm that taking into account the area and perimeter of the deformed platelet as it passes through the region of interest. Results of a typical RT‐DC measurements involving washed platelets (D) show that unstimulated deform more (ie, softer), while those treated with agonist (TRAP‐6) deform less (ie, stiffer; upper panels). Platelets preincubated with cytochalasin D and blebbistatin lead to increased deformation (ie, softer; lower panels). Data show density maps of deformation against area (μm2) from n ≥ 2500 individual platelets from a single experiment
