Fig. 2.
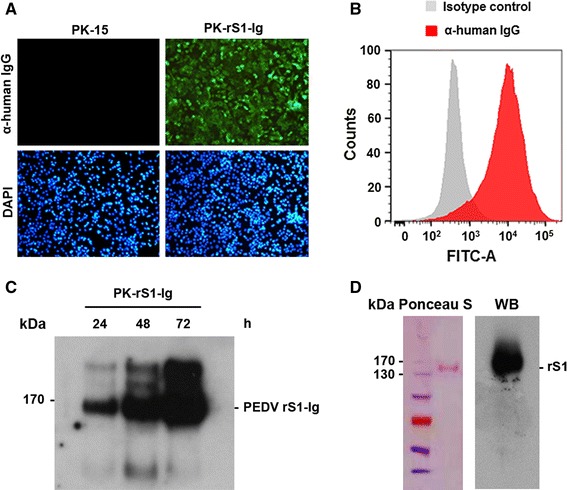
Constitutive expression of the recombinant S1 protein in PK-rS1-Ig cells. (A) Immunofluorescence assay of the rS1 protein. PK-15 or PK-rS1-Ig cells grown in a 6-well tissue culture plate were fixed with 4 % formaldehyde at 48 h post-seeding and incubated with anti-human IgG antibody (top panels). The cells were then counterstained with DAPI (bottom panels) and examined using a fluorescent microscope at 400× magnification. (B) Intracellular expression of rS1. One million cells were harvested at 48 h post-seeding and incubated with anti-human IgG antibody (red histogram) or an isotype control (white histogram) and analyzed by flow cytometry. (C) Extracellular expression of the rS1 protein. PK-rS1-Ig cells were grown in a 6-well tissue culture plate at 5 × 105 cells/well for 24, 36, and 48 h. Culture supernatants were harvested at the indicated time points and immunoprecipitated with protein A Sepharose beads. The beads were subjected to western blot analysis with anti-human IgG antibody to determine the expression level of the soluble rS1 protein. (D) Purification of the rS1 protein. The recombinant S1 protein was purified from serum-free medium of PK-rS1-Ig grown in a 6-well tissue culture plate. The purified rS1 protein was resolved in a 4–12 % gradient Bis-Tris gel and electrotransferred onto nitrocellulose paper. The membrane was stained with Ponceau S solution (left panel), destained, and blotted with antibody specific for human IgG (right panel) (color figure online)
