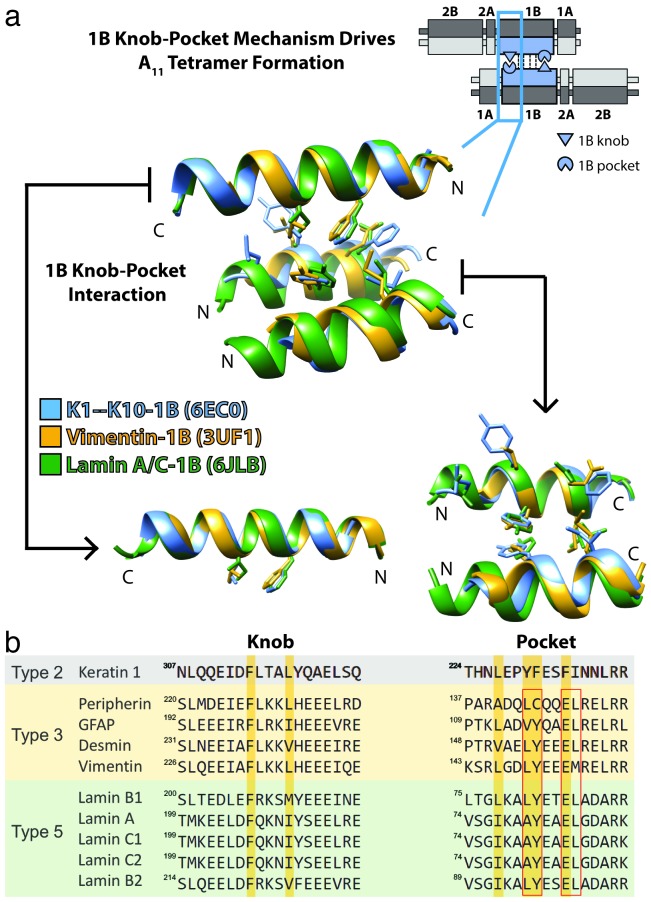
Figure 2.
Knob-pocket mechanism in helix 1B is important for A11 tetramer formation for multiple IF types. (a) For orientation, a schematic depicts a keratin A11 tetramer (upper right) and shows the position of symmetric knob-pocket interactions in the 1B helical region of the Type II keratin (blue). Below, the crystal structures of K1/K10-1B (blue, PDB ID 6EC0), vimentin-1B (gold, PDB ID 3UF1), and lamin A/C-1B (green, PDB ID 6JLB) were superposed and the knob-pocket interaction presented. There is structural conservation of the 1B knob-pocket tetramer assembly mechanism for Type II, Type III, and Type V IFs. There are no structures to date of Type IV or VI IFs. (b) Multiple sequence alignment for the knob and pocket regions of select Type II, Type III, and Type V IFs. There is complete conservation of the key phenylalanine residue in the knob. There is also a high degree of conservation of the pocket residues between Type III and Type V IFs (red boxes), which can be compared to the yellow bars for keratin 1. The structural superposition in panel (a) demonstrates the pocket is structurally conserved despite there being a variation in how the pocket is formed between heterodimeric IFs (Type I/II) and homodimeric IFs (Types III and V). The fact that heterodimeric and homodimeric IFs both utilize the 1B knob-pocket mechanism reinforces its importance to IF assembly.