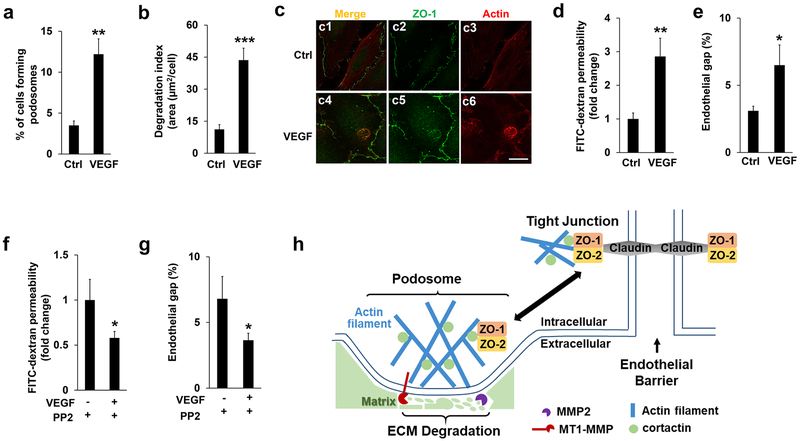
Figure 7. VEGF treatment regulates podosomal ZO proteins and endothelial barrier function.
(a-b) VEGF (50 ng/ml) were added to HBMECs for 1 hour, followed by podosome formation and ECM degradation assay as described previously. At least 9 different fields (10X objectives) were selected to calculate the percentage of podosomes formation (a) and the index of ECM degradation (b) as described previously. n=3. (c) HBMECs were immunostained by both ZO-1 and Actin antibodies in the absence (DMSO) and presence of VEGF (50 ng/ml, 1 hour). Note that VEGF treatment induced the podosomal localization of ZO-1 judged by the merged signals in c6. At least 6 different fields were selected (10 X objectives) and the podosomal ZO-1 were detected in all the cells that display podosomes, n=3. Scale bar: 10 µm. (d-e) FITC-dextran permeability assay (d) or endothelial gap formation assay (e) were applied to HMBECs in the absence (DMSO) and presence of VEGF (50 ng/ml, 1 hour). Note that VEGF treatment significantly induced endothelial barrier leakiness. (f-g) HBMECs pre-incubated with PP2 (10 µM, 1 hour) or DMSO (Ctrl) were further stimulated by VEGF treatment. Inhibition of SRC kinase by PP2 treatment further attenuated VEGF-induced endothelial barrier defects as monitored by the FITC-dextran permeability (f) and endothelial gap assay (g), respectively. n=3, *p<0.05, **p<0.01, ***p<0.01. (h) A schematic model for the role of ZO-1/2 in the cross-talk between podosomes and tight junctions in endothelial cells.