Abstract
Background
Electrochemotherapy (ECT) is a local cancer treatment based on electroporation where the electric field is used to enhance cell membrane permeability and thereby facilitating the transition of chemotherapeutic agents into the cell. For the treatment of non-melanoma skin cancer, a standard dosage of 15,000 IU/m2 bleomycin (BLM) is used. The aim of the present study was to evaluate the long-term ECT response in the group of elderly patients with non-melanoma skin cancer treated with a reduced dose of BLM in comparison to the outcome in the patients treated with the standard dose of BLM.
Patients and methods
Twenty-eight patients older than 65 years, with a total of 52 non-melanoma skin lesions were included in the study. Twelve patients (24 lesions) in the experimental group received a reduced dose of BLM (10,000 IU/m2), 16 patients (28 lesions) were treated with a standard dose of BLM (15,000 IU/m2).
Results
No statistically significant difference in tumor control was observed between both groups. In the experimental group, tumors recurred in 39.0% of treated lesions in a median follow-up time of 28 months. In the control group, the recurrence rate of treated lesions was 15.4% in a median follow-up time of 40 months.
Conclusions
ECT with a reduced dose of BLM is a feasible treatment option for elderly patients with equal efficacy to standard dose treatment and should be considered as a treatment modality in advanced aged patients with comorbidities, where overall life expectancy is poor.
Key words: electrochemotherapy, bleomycin, non-melanoma skin cancer
Introduction
Electrochemotherapy (ECT) is a local cancer treatment modality with proven antitumor efficacy in various histological types of malignant tumors.1 During an ECT procedure, electroporation is used to transiently increase cell permeability to a hydrophilic chemotherapeutic agent such as bleomycin (BLM) and cisplatin.2 Currently, intravenous administration of BLM is the most common way of drug administration utilized in ECT treatment.3, 4
ECT acts through at least three different mechanisms. The first mechanism is a direct cytotoxic effect driven by the transition of a chemotherapeutic agent into the tumor cells, which is enhanced by electroporation. ECT also has an impact on tumor blood vessels through sympathetic nerve stimulation and subsequent vasoconstriction lasting several hours. Consequently, drug washout from the tumor is delayed. Additionally, the cytotoxic effect on endothelial cells of tumor blood vessels results in the late destruction of tumor vasculature by vascular disrupting effect. The third mechanism involves immune response provoked by immunogenic cell death and enhanced tumor antigen expression.5
In the ECT treatment of non-melanoma skin cancer (NMSC) compelling results were achieved when using standard dose (15,000 IU/m2) of intravenously administrated BLM.6 According to published BLM pharmacokinetics study, an equally good antitumor response might be obtained with a reduced dose of BLM in the elderly population due to the slow elimination rate of BLM.7 Hence the updated SOP for ECT proposes de-escalation of standard dose due to high age and/or compromised creatinine clearance.3 Till today only two clinical studies have been focused on ECT effects with a reduced dose of BLM. Both compared treatment response two months after ECT and showed similar efficacy to the standard dose of BLM.8, 9 Even more, in one study authors observed faster healing time and favorable cosmetic outcomes when using the reduced dose.9
The aim of the present study was to evaluate the long-term ECT response in the group of elderly patients with NMSC treated with a reduced dose of BLM in comparison to the outcome in the patients treated with the standard dose of BLM.
Patients and methods
Study summary
The study was conducted between June 2014 and June 2019 at the Department of Otorhinolaryngology and Cervicofacial Surgery, University Medical Centre Ljubljana. The study protocol was approved by the Republic of Slovenia National Medical Ethics Committee (182/02/14 and 0120-132/2015-2). Patients were selected according to the inclusion and exclusion criteria listed in Standard Operating Procedures of ECT (SOP) and as previously reported.3, 7,9 All patients had treatment naïve and biopsy-verified primary NMSC in the head and neck region. Each patient was presented to the multidisciplinary head and neck tumor board that confirmed indication. Prior to ECT procedure a written informed consent was obtained from all patients.
The study was conducted as a nonrandomized prospective study. Patients were grouped according to BLM dose they received. The patients who were treated with a standard dose of BLM formed the control group and data from that group was used to determine BLM pharmacokinetics in elderly patients. 7 All subsequently treated patients older than 65 years received the reduced dose and formed the experimental group.
Figure 1.
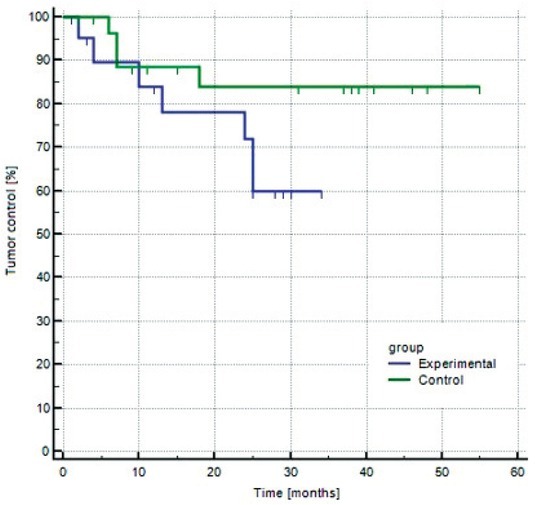
Kaplan-Meier curve of tumor control in experimental and control group.
Procedure
ECT was performed under sedation or general or local anesthesia. BLM (Bleomycin medac; Medac, Wedel, Germany) was administered as intravenous bolus injection in 2 minutes at a dose of 15,000 IU/m2 body surface area in the control group and at a dose of 10,000 IU/m2 body surface area in the experimental group (1,000 IU is equal to 1 mg of bleomycin activity). In both groups, the electric pulses were applied 8 minutes after the injection of BLM by electrodes with fixed geometry (hexagonal, needle row, or plate electrodes). Electric pulses were generated by Cliniporator Pulse Generator (IGEA, s.r.l., Carpi, Italy), as described previously.9
Follow up
Response to the treatment was evaluated according to Response Evaluation Criteria in Solid Tumors criteria, version 1.1.10 The two-month outcome has already been reported in 2018 in our previous publication.9 In this study we evaluated treatment outcome at 2, 4, 6, 12, 18, 24 months after ECT, and yearly thereafter. Minimal follow-up time of 6 months was required. Based on their presentation at three-year follow-up some patients were deemed disease free and were excluded from further follow-up at our institution. Those patients were referred to dermatologist for future yearly follow-up with instruction to report back in case of suspected recurrence of malignancy in treated area.
Statistical analysis
Statistical comparison between the control and experimental group was performed using the Mann-Whitney test (age of the patients and maximal tumor diameter) and the Chi-square test (sex, histology type, number of recurrent lesions and response to treatment). The results were analyzed using the PC SPSS, release 18.0 (SPSS, Chicago, IL) statistical package. All the tests were 2-sided, and the results were considered significant at a probability level of 5%. Kaplan-Maier plots were drawn, and the log-rang test was performed for the evaluation of longterm tumor control.
Results
Twenty-eight patients, 65 years or older, with histologically proven NMSC in the region of the head and neck, were included in the study. All the patients were treated by ECT, using intravenous bolus injection of BLM in standard (15,000 IU/m2 body surface area) or reduced (10,000 IU/m2 body surface area) dose. There were no statistically significant differences between both groups concerning the patients (age and sex) and tumors (diameter, histology type, and recurrent lesions) characteristics (P > 0.05). Details of patients and tumors treated with reduced and standard BLM doses are presented in Tables 1 and 2.
Table 1.
Patients’ characteristics, treatment procedure and response to treatment of patients included in experimental group
| Patient | Gender | Age (years) | Creatinine (μmol/L) | Total bleomycin dose (IU) | Electrodes used | Last follow up (months after ECT) | Histology | Maximum tumor size (mm) | Recurrence of tumor (months after ECT) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 86 | 84 | 20,000 | Needle row | 30 | BCC BCC |
20 8 |
|
| 2 | M | 67 | 108 | 24,000 | Needle row | 29 | BCC BCC |
10 8 |
|
| 3 | M | 80 | 106 | 21,000 | Needle row | 20 | BCC | 30 | 10 |
| 4# | M | 82 | 66 | 20,000 | Needle row | 2 * | SCC SCC SCC |
10 6 10 |
|
| 5 | M | 92 | 88 | 20,000 | Needle row | 25 | BCC BCC BCC |
25 15 15 |
25 25 |
| 6 | M | 79 | 67 | 20,000 | Plate | 34 | BCC BCC BCC BCC |
80 20 20 10 |
24 |
| 7 | F | 78 | 83 | 20,000 | Needle row | 30 | BCC | 15 | |
| 8# | M | 76 | 144 | 21,000 | Needle row | 6 * | SCC SCC SCC |
20 27 35 |
|
| 9 | F | 86 | 74 | 17,500 | Needle row | 19 * | BCC | 25 | |
| 10 | M | 80 | 81 | 21,000 | Needle row | 28 | BCC | 10 | 13 |
| 11 | M | 85 | 42 | 20,000 | Needle row | 24 * | BCC | 50 | 3 |
| 12 | M | 83 | 94 | 19,000 | Needle row | 28 | BCC SCC |
7 7 |
4 |
BCC = basal cell carcinoma; ECT = electrochemotherapy; F = female; M = male; SCC = squamous cell carcinoma; * = patient deceased; # = excluded from analysis
Table 2.
Patients’ characteristics, treatment procedure and response to treatment of patients included in control group
| Patient | Gender | Age (years) | Creatinine (μmol/L) | Total bleomycin dose (IU) | Electrodes used | Last follow-up (months after ECT) | Histology | Maximum tumor size (mm) | Recurrence of tumor (months after ECT) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 65 | 68 | 30,000 | Needle row | 39 | BCC | 22 | |
| 2 | F | 74 | 86 | 26,000 | Needle row | 55 | BCC | 15 | |
| 3 | M | 83 | 129 | 28,000 | Plate | 19 * | BCC | 12 | |
| BCC | 32 | 7 | |||||||
| BCC | 10 | 7 | |||||||
| 4 | M | 81 | 78 | 27,000 | Needle row | 37 | BCC | 10 | |
| BCC | 5 | ||||||||
| BCC | 6 | ||||||||
| BCC | 4 | ||||||||
| 5 | F | 82 | 73 | 24,000 | Plate | 41 | BCC | 9 | 6 |
| BCC | 5 | ||||||||
| BCC | 11 | ||||||||
| 6# | M | 88 | 142 | 30,000 | Plate | 2 | BCC | 39 | |
| 7 | F | 69 | 84 | 23,000 | Finger | 46 | BCC | 15 | |
| 8 | F | 82 | DM | 24,000 | Hexagonal | 41 | BCC | 50 | |
| BCC | 7 | ||||||||
| BCC | 20 | 18 | |||||||
| 9 | F | 89 | 69 | 23,000 | Plate | 48 | BCC | 6 | |
| BCC | 5 | ||||||||
| BCC | 6 | ||||||||
| 10 | M | 65 | DM | 27,000 | Plate | 31 | BCC | 24 | |
| 11# | F | 70 | DM | 27,000 | Plate | 4 | BCC | 7 | |
| 12 | M | 78 | DM | 23,000 | Plate | 46 | BCC | 15 | |
| 13 | F | 74 | 52 | 24,000 | Needle row | 38 | BCC | 15 | |
| 14 | F | 89 | 54 | 25,000 | Plate | 48 | BCC | 21 | |
| BCC | 22 | ||||||||
| 15 | M | 67 | DM | 30,000 | Plate | 11 | SCC | 25 | |
| 16 | M | 85 | 100 | 24,000 | Needle row | 15 * | SCC | 45 |
BCC = basal cell carcinoma; ECT = electrochemotherapy; F = female, M = male; SCC = squamous cell carcinoma; * = patient deceased; # = excluded from analysis
The experimental group consisted of 12 patients (10 men and 2 women; median age 81 years; range 67-92 years) with 24 lesions (17 BCCs, 7 SCCs), 18 (75%) tumors were treatment naïve. The largest tumor diameter ranged from 6 mm to 80 mm (median 21 mm). In control group 16 patients (8 men and 8 women; median age 78 years; range 65-89 years) had 28 lesions (25 BCCs; 3 SCCs), 24 (86%) tumors were treatment naïve. The largest tumor diameter ranged from 4 mm to 50 mm (median 17 mm).
The complete response rates two months after ECT were observed in control and experimental in 96% and 100%, respectively.9 All patients were further followed up at regular visits. In the experimental group, two patients died before six months follow up. One died due to the systemic dissemination of previously treated melanoma. The cause of death in another patient was an underlying disease, not related to skin cancer or ECT treatment. These two patients were excluded from the study. Out of 12 patients in the experimental group, 10 (83.0%) were evaluable at 6 months or longer. Median longterm follow up was 28 months in the experimental group (average 24.9 ± 7.4 months). Tumor recurred in 6/10 (60.0%) patients and in 7/18 (39.0%) tumors in median recurrence time 18.5 months (average 26.9 ± 9.0 months).
In the control group, 2/16 patients were excluded from the study. One died 4 months after the treatment due to other causes than cancer. The other patient did not come to a follow-up visit at 6 months and was lost to further follow-up. Fourteen patients (88%) were evaluable at 6 months or longer. Median long-term follow up was 40 months (average 36.0 ± 14.5 months). The recurrence was observed in 3/14 patients (21.4%) and in 4/26 nodules (15.4%) in a median time of 7.0 months (average 9.4 ± 5.6 months).
Overall, 6/28 (21%) patients included in the study died during follow-up due to other comorbidities. In neither of them the cause of death was related to NMSC or ECT treatment. After statistical analysis no significant difference in recurrence rate was observed between groups (Logrank test P=0,104).
No statistically significantly elevated levels of creatinine was detected neither in control (85.0 ± 28.6 μmol/L) nor in experimental group (86.4 ± 25.6 μmol/L, p > 0.1).
Discussion
Until now, only a few studies evaluated the longterm efficacy of ECT treatment in NMSC in the head and neck region. Kristiansson et al. reported on 71% control rate in a case series of 7 patients with NMSC treated with ECT after a median follow up of 119 months.11 In the most comprehensive study regarding long–term follow up after ECT treatment of BCCs, 5-year recurrence rate of local and locally advanced BCC was 20% and 38%, respectively.12 It should be emphasized that ECT in these studies was performed according to the first version of SOP, thus standard dose (15,000 IU/m2) of intravenous administrated BLM was used.13 To the best of our knowledge, our clinical study is the only one where long-term effectiveness of ECT in NMSC in the head and neck region after the intravenous administration of a reduced dose of BLM was evaluated and was found to be equal to the effectiveness of ECT with standard dose.
The efficacy of a reduced BLM dose in elderly patients was confirmed in clinical studies after the identification of the main parameters of BLM pharmacokinetics.7, 8, 9 Ageing is related to the impairment of body functions, e.g., impaired renal function, and to a reduction in lean body mass. Both changes lead to reduced clearance of water-soluble drugs (such as BLM) and reduced volume of their distribution. Hence, the plasma concentration of BLM is higher than in younger adults. Thus, the use of the standard dose of BLM in elderly patients could lead to prolonged healing time and a more prominent inflammatory response, as a result of exceeded optimal concentrations of BLM. The rationale for dose de-escalation in elderly patients lays in diminishing possible systemic side effects (e.g., lung fibrosis), improving local healing and keeping total BLM dose as low as possible, especially in circumstances when multiple sessions of ECT are expected.8, 9 According to our previous study complete response rate two months after ECT was almost 100% when using both standard (15,000 IU/m2) or reduced (10,000 IU/m2) BLM dose in elderly patients with NMSC.9 One of the major drawbacks of that study was too short observation time for adequate assessment of clinical response. This is especially important in BCCs because they are slow-growing tumors and it often takes months to years for a tumor to relapse after initial treatment.14
After statistical analysis no significant differences were observed between control and experimental groups regarding clinical or tumor parameters. In the control group, 4 of 26 tumors recurred in the median follow up time of 7.0 months. In the experimental group of patients, 7 out of 18 tumors recurred in the median follow up time of 18.5 months. After statistical analysis, no significant differences in long term tumor control between groups were observed (p=0.104). This confirms our hypothesis that long-term tumor control could be achieved with a lowered BLM dose. Our finding is in concordance with results of previous clinical trials on the elderly population, that suggest equal efficacy of reduced dose two months after the treatment even though the concentration of BLM around the tumor immediately after electroporation is lower compared to the standard dose.8, 9 These findings can be explained with the pharmacokinetics of BLM in elderly patients, where higher concentrations are achieved due to reduced BLM clearance by the kidney and by the reduced volume of distribution.7
Although differences in the long-term tumor control between both groups were not statistically significant, we observe a trend towards a recurrence of BCCs in the experimental group. We might speculate that further studies with a larger cohort of patients could show a lower tumor control of BCCs treated with reduced dose, as a result of reduced local inflammatory response. One of the antitumor mechanisms of ECT involves immune response, provoked by immunogenic cell death and enhanced tumor antigen expression.5 This might be especially important in the treatment of BCCs since these tumors have the greatest mutational burden among all human cancers.15 Consequently, numerous tumor antigens provoke immune system, which is the most probable reason for the less aggressive nature of BCCs.15 Taking these facts into consideration, less prominent local immune response after reduced dose ECT might lead to a lower tumor control of BCCs.
The overall long-term tumor control rate at the end of the study was 79%. Deaths were correlated with underlying disease and not with NMSC progression or side effects after ECT. It is important to emphasize that although long term tumor control was not achieved in all patients, reducing possible side effects and consequent quality of life in patients with short life expectancy is of paramount importance. Thus, a reduced BLM dose in ECT should be considered as a treatment modality in elderly patients with comorbidities, where overall life expectancy is poor.
One of the future perspectives of ECT should be an orientation towards individual patients and tumors. For example, some studies have already shown that differences between tumor vascularization have an impact on BLM pharmacokinetics and therefore on antitumor response.16 The exact dose to achieve maximal antitumor response with minimal side effects should be determined according to tumor histology and patients’ overall health. Hence, in Updated Standard Operating Procedures for ECT a compromised creatinine level was proposed as a guide for using a reduced dose of BLM.3 In our study average creatinine levels during ECT procedure were normal in both groups. It should be noted that the production of creatinine is decreased due to age-related reduction in skeletal muscle mass; thus, plasma creatinine level is an inaccurate indicator of glomerular filtration rate in the elderly population, where the reduction of lean body mass is prominent.7 We presume that creatinine is not a reliable marker in applying a reduced dose of BLM in the group of elderly patients and it must be interpreted in concordance with other methods such as bioelectrical impedance analysis, which is used for body composition measurements.
We are aware that a low number of patients in our study is one of the drawbacks of the study. Even though this study raises some important clinical questions, which need to be addressed. In the future, randomized studies with a larger cohort of patients, treated with reduced doses of BLM, are needed to evaluate the appropriate indications and clinical significance of de-escalated dose BLM in ECT treatment.
Acknowledgement
This research was funded by the Slovenian Research Agency, grant number P3-0003 and project J3-9269.
Disclosure
No potential conflicts of interest were disclosed.
References
- 1.Mali B, Jarm T, Snoj M, Sersa G, Miklavcic D. Antitumor effectiveness of electrochemotherapy: A systematic review and meta-analysis. EJSOl. 2013;39:4–16. doi: 10.1016/j.ejso.2012.08.016. [DOI] [PubMed] [Google Scholar]
- 2.Mir LM, Orlowski S, Belehradek J, Paoletti C. Electrochemotherapy potentiation of antitumour effect of bleomycin by local electric pulses. EJSO. 1991;27:68–71. doi: 10.1016/0277-5379(91)90064-k. [DOI] [PubMed] [Google Scholar]
- 3.Gehl J, Sersa G, Matthiessen LW, Muir T, Soden D, Occhini A. Updated standard operating procedures for electrochemotherapy of cutaneous tumours and skin metastases. Acta Oncol. 2018;57:874–82. doi: 10.1080/0284186X.2018.1454602. [DOI] [PubMed] [Google Scholar]
- 4.Campana LG, Clover AJ, Valpione S, Quaglino P, Gehl J, Kunte C. Recommendations for improving the quality of reporting clinical electro-chemotherapy studies based on qualitative systematic review. Radiol Oncol. 2016;50:1–13. doi: 10.1515/raon-2016-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campana LG, Miklavčič D, Bertino G, Marconato R, Valpione S, Imarisio I. Electrochemotherapy of superficial tumors – Current status: Basic principles, operating procedures, shared indications, and emerging applications. Semin Oncol. 2019;46:173–91. doi: 10.1053/j.seminoncol.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 6.Bertino G, Sersa G, De Terlizzi F, Occhini A, Plaschke CC, Groselj A. European Research on Electrochemotherapy in Head and Neck Cancer (EURECA) project: results of the treatment of skin cancer. Eur J Cancer. 2016;63:41–52. doi: 10.1016/j.ejca.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 7.Groselj A, Krzan M, Kosjek T, Bosnjak M, Sersa G, Cemazar M. Bleomycin pharmacokinetics of bolus bleomycin dose in elderly cancer patients treated with electrochemotherapy. Cancer Chemother Pharmacol. 2016;77:939–47. doi: 10.1007/s00280-016-3004-z. [DOI] [PubMed] [Google Scholar]
- 8.Rotunno R, Campana LG, Quaglino P, De Terlizzi F, Kunte C, Odili J. Electrochemotherapy of unresectable cutaneous tumours with reduced dosages of intravenous bleomycin: analysis of 57 patients from the International Network for Sharing Practices of Electrochemotherapy registry. J Eur Acad Dermatol Venereol. 2018;32:1147–54. doi: 10.1111/jdv.14708. [DOI] [PubMed] [Google Scholar]
- 9.Groselj A, Bosnjak M, Strojan P, Krzan M, Cemazar M, Sersa G. Efficiency of electrochemotherapy with reduced bleomycin dose in the treatment of nonmelanoma head and neck skin cancer: Preliminary results. Head Neck. 2018;40:120–5. doi: 10.1002/hed.24991. [DOI] [PubMed] [Google Scholar]
- 10.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 11.Kristiansson S, Reizenstein J, von Beckerath M, Landström F. Long-term follow-up in patients treated with electrochemotherapy for non-melanoma skin cancer in the head and neck area. Acta Otolaryngol. 2019;139:195–200. doi: 10.1080/00016489.2018.1543950. [DOI] [PubMed] [Google Scholar]
- 12.Campana LG, Marconato R, Valpione S, Galuppo S, Alaibac M, Rossi CR. Basal cell carcinoma: 10-year experience with electrochemotherapy. J Transl Med. 2017;15:122. doi: 10.1186/s12967-017-1225-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mir LM, Gehl J, Sersa G, Collins CG, Garbaya JR, Billarda V. Standard operating procedures of the electrochemotherapy: Instructions for the use of bleomycin or cisplatin administered either systemically or locally and electric pulses delivered by the CliniporatorTM by means of invasive or non-invasive electrodes. EJC Suppl. 2006;4:14–25. doi: 10.1016/j.ejc-sup.2006.08.003. [DOI] [Google Scholar]
- 14.Bartoš V, Pokorný D, Zacharová O, Haluska P, Doboszová J, Kullová M. Recurrent basal cell carcinoma: a clinicopathological study and evaluation of histomorphological findings in primary and recurrent lesions. Acta Dermatovenerol Alp Pannonica Adriat. 2011;20:67–75. PMID: 21993704. [PubMed] [Google Scholar]
- 15.Jayaraman SS, Rayhan DJ, Hazany S, Kolodney MS. Mutational landscape of basal cell carcinomas by whole-exome sequencing. J Invest Dermatol. 2014;134:213–20. doi: 10.1038/jid.2013.276. [DOI] [PubMed] [Google Scholar]
- 16.Groselj A, Kranjc S, Bosnjak M, Krzan M, Kosjek T, Prevc A. Vascularization of the tumours affects the pharmacokinetics of bleomycin and the effectiveness of electrochemotherapy. Basic Clin Pharmacol Toxicol. 2018;123:247–56. doi: 10.1111/bcpt.13012. [DOI] [PubMed] [Google Scholar]


