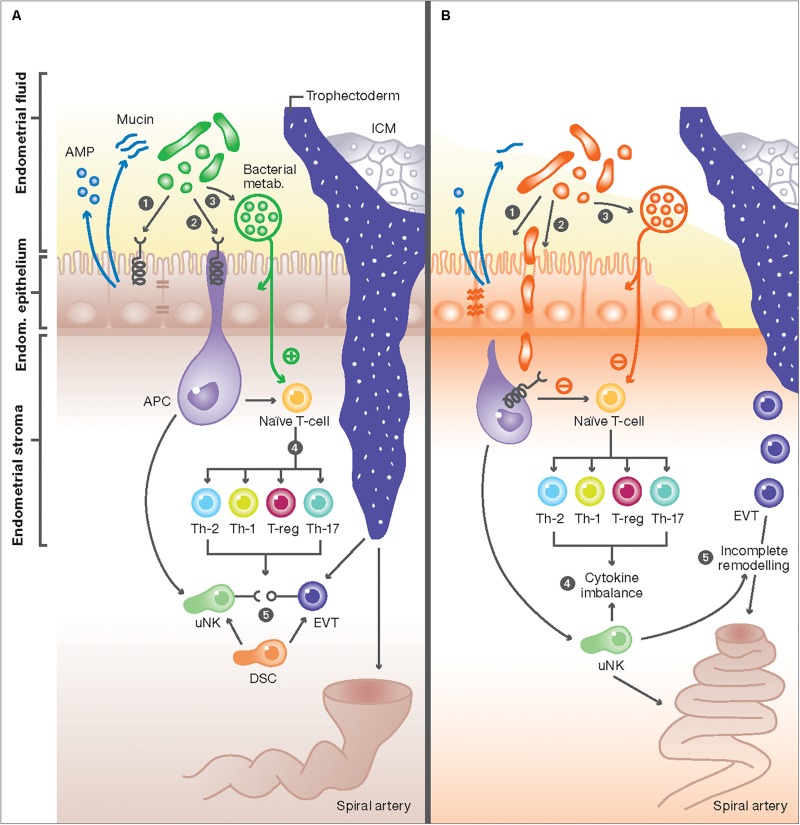
FIGURE 1.
The interaction between the endometrial microbiome and local immune mediators. (A) In healthy women, commensal bacterial communities interact with immune cells with at the feto-maternal interface through three potential mechanisms: (1) Commensal bacteria (green) maintain a healthy physical barrier by stimulating the production of different antimicrobial peptides (AMP) from endometrial cells and preserving epithelial tight junctions and stable mucus production. (2) Once encountered by immune cells in the endometrium, e.g., antigen presenting cells (APC), commensal bacteria trigger signal transduction via pattern recognition receptors (PRR) through their pathogen-associated molecular patterns (PAMPs). (3) Commensal bacteria can also produce metabolites, such as polysaccharides and short-chain fatty acids (SCFAs), that potentially affect immune responses in endometrial epithelial cells and T-cells, or alter the endometrial fluid pH to produce a competitive niche microenvironment against pathogenic bacteria. These mechanisms result in activation of uterine NK (uNK) cells and the development of specific subsets of T-cells, characterized by high number of regulatory T-cells (Treg), low number of Th-17, and a switch from the Th1 to Th2 cytokine production (4). The interaction of activated uNK cells (KIR receptors) with HLA-C and -G from extravillous trophoblasts (EVT) from the implanting embryo will also promote EVT invasion, stromal matrix degradation, angiogenesis and ultimately the remodeling of maternal spiral arteries (5). These adaptive changes ensure an immunotolerant milieu for the semi-allogenic fetus and are essential steps essential step in normal placentation. (B) Disturbance of the normal endometrial microbiome can negatively impact the implantation process: (1) First, the dominance of non-commensal bacterial communities could weaken the integrity of the endometrial mucosal barrier by affecting the epithelial tight junctions and reducing AMP and mucin secretion. (2) This in turn will further weaken host defense mechanisms and allow pathogens to enter the endometrial stroma and elicit a profound immune reaction from APC and other immune cells harboring pattern recognition receptors (PRR). (3) Aberrant stimulation of T-cells, either directly from invading pathogens breaching the mucosal barrier or indirectly from absorbed bacterial products results in disbalance in cytokine production in favor of the pro-inflammatory Th-1 types, predominated by TNF-a, IFN, IL-2, and IL-10 (4). Aberration in uNK cell maturation, either primary or secondary to shallow EVT invasion, is a possible link between disturbed endometrial microbiome and incomplete remodeling of maternal spiral arteries, characteristic of the great obstetric syndromes (5).