Fig. 1.
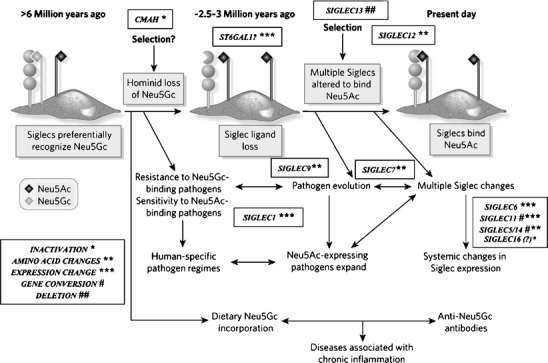
Suggested scenario for multiple changes in sialic acid biology during human evolution. The initial loss of Neu5Gc expression during human evolution could have occurred randomly, or due to selection by a pathogen that preferentially recognized Neu5Gc on cell surfaces (e.g., a form of hominid malaria, or a bacterial toxin). Regardless of the reason, the resultant loss of Neu5Gc-binding sites for some CD33rSiglecs should have generated unusual immune activation, and further selection was likely required to allow adjustment for binding of Neu5Ac in some Siglecs, with elimination or loss of binding by others. Thus, all the other human specific changes in sialic acid biology discussed in the text could have resulted from adjustments to the original event of CMAH inactivation. Of course, other scenarios are possible. A non-genetic complexity is also indicated, in which dietary Neu5Gc can accumulate in human tissues in the face of an anti-Neu5Gc response, potentially facilitating diseases associated with chronic inflammation. (Modified from Varki A. Nature 446: 1023, 2007). Note that while CD33rSiglecs are shown as binding to sialic acids on the same cell surface, they can also potentially detect high densities of sialic acids on other cell surfaces or on Neu5Ac-expressing pathogens (which are common in humans)
