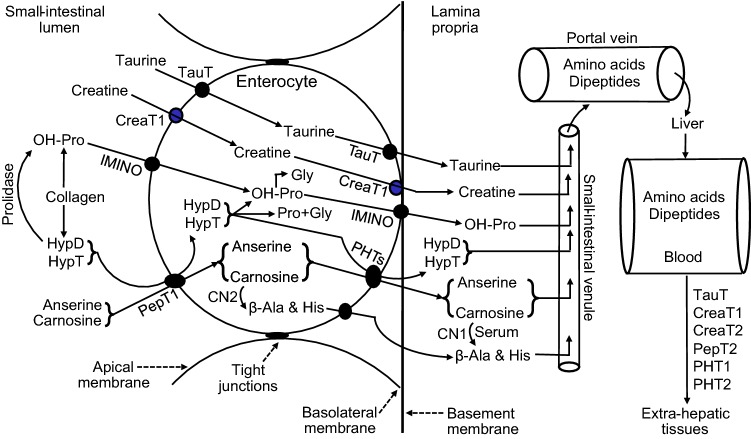
Fig. 1.
Absorption of taurine, creatine, carnosine, anserine, and 4-hydroxyproline by the human small intestine and the transport of the nutrients in blood. Dietary collagen is hydrolyzed by proteases, peptidases and prolidase to free amino acids as well as 4-hydroxyproline and its peptides. Dietary taurine, creatine, carnosine, anserine, and 4-hydroxyproline are taken up by the enterocyte across its apical membrane via specific transports. Inside the cell, taurine, creatinine and anserine are not degraded, some of the 4-hydroxyproline-containing peptides are hydrolyzed to 4-hydroxyproline and its peptides, some 4-hydroxyproline is oxidized to glycine, and carnosine undergoes limited catabolism. Taurine, creatine, carnosine, anserine, and 4-hydroxyproline, as well as the products of carnosine hydrolysis (β-alanine and histidine) exit the enterocyte across its basolateral membrane into the lamina propria of the intestinal mucosa via specific transporters (Wu 2013). The absorbed nutrients are transported in blood in the free forms for uptake by extra-intestinal tissues via specific transporters. β-Ala β-alanine, CAT cationic amino acid transporter, CN1 carnosinase-1 (serum carnosinase), CN2 carnosinase-2 (tissue carnosinase), CreaT1 creatine transporter-1, CreaT2 creatine transporter-2, GAT γ-aminobutyrate transporter, HypD 4-hydroxyproline-containing dipeptides, HypT 4-hydroxyproline-containing tripeptides, OH-Pro 4-hydroxyproline, PAT1 proton-(H+-coupled) and pH-dependent but Na+- and Cl−-independent transporter for taurine (low-affinity, high-capacity transporter), PepT1 peptide transporter-1, PepT2 peptide transporter 2, PHT1/2 peptide/histidine transporters 1 and 2, TauT taurine transporters. Note that the distribution of PHT1/2 in tissues is species-specific in that human skeletal muscle expresses PHT1 but no PHT2, whereas mouse skeletal muscle expresses both PHT1 and PHT2