Abstract
The objective was to describe the probability of Cryptosporidium parvum fecal oocyst shedding at different magnitudes of exposure, the pattern of fecal shedding over time, and factors affecting fecal shedding in dairy calves. Within the first 24 h of life, 36 calves were experimentally challenged with C. parvum oocysts at one of four possible magnitudes of oral exposure (1 × 103, 1 × 104, 1 × 105, and 1 × 106 oocysts), and 7 control calves were sham dosed. Fecal shedding occurred in 33 (91.7 %) experimentally challenged calves and in none of the control calves. There was a difference in the log-total number of oocysts counted per gram of feces dry weight among the four exposure groups; calves with the lowest magnitude of exposure (1 × 103 oocysts) shed less than the other three groups. At higher magnitudes of exposure, there was more variability in the range of fecal oocyst shedding. There was an inverse relationship between the log-total amount of oocysts counted per gram of feces dry weight and the number of days to the onset of fecal shedding per calf, i.e., the more time that elapsed to the onset of fecal shedding, the fewer oocysts that were shed. The pattern of fecal shedding over time for all calves shedding oocysts was curvilinear; the number of oocysts increased with time, reached a peak, and declined. Therefore, the dynamics of oocyst shedding can be influenced in part by limiting exposure among calves and delaying the onset of fecal oocyst shedding.
Keywords: Dose Group, Serum Total Protein, Cryptosporidiosis, Oral Exposure, Cryptosporidium Parvum
Introduction
The Apicomplexa protozoan parasite genus Cryptosporidium is comprised of over 20 species to date (Fayer 2004). Transmission of Cryptosporidium is most often by the fecal–oral route via contaminated water, food, or fomites or by direct ingestion of infected feces. In cattle, Cryptosporidium bovis, Cryptosporidium andersoni, and Cryptosporidium parvum are species of particular importance. Among these three species, C. bovis and C. andersoni are prevalent in post-weaned and adult cattle, respectively, and are not important zoonoses (Fayer et al. 2007). In contrast, C. parvum is an important zoonosis, is the most pathogenic species in cattle, and is prevalent in pre-weaned calves (Fayer et al. 2007; Lindsay et al. 2000; Robinson et al. 2006; Trotz-Williams et al. 2006; Santin et al. 2004). C. parvum infects many mammals and is globally recognized as a significant cause of diarrhea in people and cattle (Feltus et al. 2006; Xiao and Feng 2008). Cryptosporidium hominis, which is host adapted to humans, is also an important cause of diarrhea in people and was the cause of the largest water-borne disease outbreak ever recorded, impacting approximately 403,000 citizens of Milwaukee, WI, USA in 1993 (Chappell et al. 2006; MacKenzie et al. 1995; Morgan-Ryan et al. 2002).
Cryptosporidiosis is the clinical syndrome of fever, diarrhea, and large volumes of fluid loss from the gastrointestinal tract (DuPont et al. 1995; Fayer 2004). The severity of this diarrhea and its subsequent dehydration influence morbidity and mortality in people and animals (Anderson 1998; O’Handley et al. 1999). Treatment of the illness poses a challenge as there is not a consistently effective chemotherapeutic agent for use in animals or in people (Amadi et al. 2002; Anderson and Curran 2007; Graczyk et al. 2011; Harp and Goff 1998; Mead 2002). For example, nitazoxanide has shown efficacy in immune-competent but not immune-compromised children (Amadi et al. 2002). In experimentally infected dairy calves, treatment with nitazoxanide reduces the duration of fecal oocyst shedding and improves clinical signs; however, there are no animal-labeled formulations at this time (Ollivett et al. 2009). Animal-labeled formulations of halofuginone are available. While halofuginone has been shown to improve clinical signs by delaying the onset of diarrhea and reducing fecal oocyst shedding, it does not prevent diarrhea and fecal shedding entirely (Jarvie et al. 2005).
In neonatal dairy calves, C. parvum is one of the leading causes of cryptosporidiosis and contributes to calf-hood diarrhea (Lefay et al. 2001; Trotz-Williams et al. 2007). Infection and disease most commonly occur in calves between 1 and 4 weeks of age and usually last for approximately 2 weeks (de Graaf et al. 1999; O’Handley et al. 1999; Xiao and Herd 1994). In young animals, during the first few days of natural infection, large numbers of oocysts may be shed in the feces. For example, a naturally infected 6-day-old calf that sheds oocysts for 6 days may produce in excess of 3 × 1010 oocysts (Nydam et al. 2001). Excreted oocysts are readily infective and can survive for several weeks to months in the environment and likely cause infection when calves are born into contaminated maternity pens or placed in contaminated housing (Anderson 1998). Therefore, the extent of calf exposure to infective oocysts may be associated with the degree of environmental contamination. There is currently little published information on this relationship in calves, nor is there literature describing the pattern of fecal oocyst shedding over time as it relates to the magnitude of exposure.
Currently, the relationship between the magnitude of oral exposure and fecal oocyst shedding is not well described in calves. In this study, we experimentally challenged neonatal dairy calves with C. parvum oocysts and reported the probability of fecal oocyst shedding, as well as the pattern of fecal shedding over time and the factors affecting this pattern.
Materials and methods
Challenge model
Calves used in this study were cared for in compliance with the Institutional Animal Care and Use Committee of Cornell University. This randomized, double-blinded study was performed at the College of Veterinary Medicine, Cornell University (Ithaca, NY, USA) from June 2007 to August 2010. Forty three calves were purchased at birth from a local dairy farm and enrolled in the study as they were born. Control calves (n = 7) were enrolled concurrently with test calves (n = 36). At least one study author attended all calvings. The perineum of the dam was thoroughly cleaned with povidone-iodine scrub, and calves were caught on single-use plastic sheets to prevent on-farm manure contamination. Immediately after birth, a physical examination was performed, and an identification tag was placed in the right ear. All calves were fed 2–4 l of maternal colostrum or ≥100 g IgG per dose commercial colostrum replacer (Bovine IgG, Colostrum Replacement, Land O’ Lakes Inc.) within the first 4 h of life. The calves were then transported from the source farm to Cornell University.
At Cornell University, all calves were individually housed in concrete box stalls so as to prevent any contact between calves. Blood samples were collected from each calf within 24–48 h of life, and the serum total protein was measured in order to assess adequacy of passive transfer. Calves were fed commercial 22 % protein/20 % fat non-medicated milk replacer (Nursing Formula NT Calf Milk Replacer, Land O’Lakes Inc.) with at least 0.68 kg of dry matter per day, split into two feedings, for the duration of the study, and water was provided ad libitum. Calves received an oral challenge of C. parvum oocysts within the first 24 h of life. Each calf was inoculated with either zero oocysts (control) or one of four possible dose magnitudes of a genotyped field strain of C. parvum oocysts. At enrollment, both control and test calves were randomized to dose group by a number generator. Seven calves received zero oocysts, 13 calves received 1 × 103 oocysts, 7 calves received 1 × 104 oocysts, 8 calves received 1 × 105oocysts, and 8 calves received and 1 × 106 oocysts. Study personnel responsible for data collection and analysis were blinded to dose group. Calves were enrolled in the study for 16–21 days.
Control calves (n = 7) were housed in the same facility as test calves, sham dosed, and managed as if they were test calves in order to maintain blinding. Control calves also served as sentinels for cross contamination from test calves and to help maintain quality assurance in data collection and husbandry practices. To prevent cross contamination, calves were fed and bedded in the same order (youngest to oldest) each day, each calf stall had dedicated equipment and supplies, and all study personnel used single-use personal protective equipment when entering each calf stall.
The oocysts used to dose the calves were purified using a procedure previously described (Jenkins et al. 1997). In brief, feces were collected from naturally infected 6- to 14-day-old calves from a separate commercial dairy operation and processed by continuous-flow differential density flotation. They were stored at 4 °C in suspension with 100 U of penicillin G sodium per milliliter, 100 mg of streptomycin sulfate per milliliter, and 0.25 mg of amphotericin B per milliliter for up to 2 months or until needed. The oocyst DNA was genotyped as C. parvum by sequence and restriction fragment length polymorphism analysis via amplification of the small subunit (SSU) rRNA gene in a nested polymerase chain reaction (PCR) as described previously (Jiang et al. 2005). In brief, the primary PCR step amplifies a fragment of approximately 1,325 base pairs, whereas the secondary PCR step results in a fragment of approximately 823 base pairs. In the primary PCR, the following forward and reverse primers were used, respectively: 5′-TTCTAGAGCTAATACATGCG-3′ and 5′-CCCATTTCCTTCGAAACAGGA-3′. In the secondary PCR, the following forward and reverse primers were used, respectively: 5′-GGAAGGGTTGTATTTATTAGATAAAG-3′ and 5′-CTCATAAGGTGCTGAAGGAGTA-3′.
Before inoculation, oocysts were first cleaned for 1 min in 0.6 % sodium hypochlorite to inactivate viruses and bacteria co-purified with the oocysts, then washed four times with phosphate-buffered saline to remove the sodium hypochlorite, quantified using a hemocytometer, and finally, viability determined using a dye permeability assay as described previously (Campbell et al. 1992; Anguish and Ghiorse 1997; Jenkins et al. 1997). Viable oocysts were the sum of 49,6-diamidino-2-phenylindole-negative (DAPI−), propidium iodide-negative (PI−) oocysts, and DAPI-positive (DAPI+) PI-oocysts; DAPI + PI + oocysts were considered inactivated (Jenkins et al. 1999). Oocysts used for dosing were at least 87 % viable. Doses were calculated based on the percent viable. Each dose was administered in a 5-ml suspension of C. parvum oocysts in reverse osmosis water via the rigid portion of an oroesophageal feeding tube, followed by 120 ml of water to ensure all of the oocyst suspension was delivered to the calf
Fecal sample analysis
Quantitative analysis of C. parvum oocysts in the fecal samples collected was performed using Merifluor Crypto/Giardia immunofluorescence antibody detection reagent from Meridian Diagnostics (Cincinnati, OH, USA) (Xiao and Herd 1993). The immunofluorescence procedure was modified from the kit instructions. Briefly, a 0.10-g portion of feces was mixed into 10 ml of PBS (pH = 7.4) in a 15-ml conical centrifuge tube. Then, 100 μl of the mixture was removed, and 5 μl of Merifluor immunofluorescence antibody reagent was added. The solution was vortexed and incubated in the dark at room temperature for at least 30 min and stored at 4 °C until examination. Following incubation, 10.5 μl of the sample was placed on a slide and covered with a coverslip. The ×20 objective on a fluorescent compound binocular microscope (460–490 wavelength fluorescent compound binocular microscope Olympus BX41, Olympus America Inc., Center Valley, PA, USA) was used to count the number of oocysts observed. The number of oocysts observed in 10.5 μl was then multiplied by 10,000 to give the number of oocysts per gram of feces. This count was standardized by the dry weight percentage. Dry weight analysis of fecal samples was obtained by taking a 10- to 20-g portion of each original fecal sample, drying it at 108 °C for a minimum of 24 h (Thermolyne Mechanical Oven, Barnstead International, Dubuque, IA, USA), then weighing it directly (Precision Standard Scale, Ohaus Corporation, Pine Brook, NJ, USA) (Bellosa et al. 2011).
Data analysis
Data were analyzed using descriptive and inferential methods. For each magnitude of exposure, the probability of shedding on a given day after challenge was estimated using the Kaplan–Meier product limit method (Kaplan and Meier 1958). Analysis of variance was used to evaluate whether or not there were differences among exposure groups with respect to the onset, cessation, and duration of fecal oocyst shedding and the total amount of fecal oocysts counted per gram of feces dry weight that could be attributed to the differences in the four exposure groups. The total amount of fecal oocysts counted per gram of feces dry weight was normalized via log transformation. Post-hoc analysis of the total amount of fecal oocysts counted per gram of feces dry weight across the four dose groups was carried out with Tukey’s HSD. The relationship between the log-total number of oocysts counted per gram of feces dry weight and the log-dose, total protein (g/dl), and number of days until the onset of fecal shedding were evaluated using linear regression methods. The pattern of fecal shedding over time was also evaluated using linear regression methods. Data were analyzed using JMP 9.0 (SAS Institute Inc. 1989–2007).
Results
Among the 43 calves enrolled in the study, 7 were control calves, and 36 were experimentally challenged with C. parvum oocysts. Calves had a mean serum total protein (g/dl) of 5.3 ± 0.35 (95 % CI 5.1–5.4). There was no difference in serum total protein (g/dl) among the four dose groups; each group attained adequate passive transfer (>5.0 g/dl). None (0 %) of the seven control calves shed oocysts or had clinical signs consistent with cryptosporidiosis. Thirty three (91.7 %) of the experimentally challenged calves shed C. parvum oocysts in their feces, developed diarrhea, and exhibited other clinical signs associated with cryptosporidiosis. The mean total oocysts counted per gram of feces dry weight for all calves that shed oocysts (n = 33) was 1.05 × 108 (95 % CI 5.03 × 107−1.60 × 108).
The average number of days until the onset of fecal oocyst shedding post-challenge was 8.6 ± 1.5 (n = 33). Figure 1 shows the probability of onset of fecal oocyst shedding on any given day post-challenge for each dose group. Among the four dose groups, the number of days until the onset of fecal oocyst shedding post-challenge tended to be influenced by dose magnitude (p = 0.17). Among the 33 calves that shed oocysts in their feces, the earliest onset of shedding was at 4 days post-challenge, and the latest onset of shedding was at 12 days post-challenge (Table 1). Both of these individuals were dosed with 1 × 103 oocysts (Fig. 1). Calves that were experimentally challenged with 1 × 103 and 1 × 104 C. parvum oocysts had a median time to onset of fecal shedding post-challenge of 8 days (Table 1). Calves that were experimentally challenged with 1 × 105 and 1 × 106 C. parvum oocysts had a median time to onset of fecal shedding post-challenge of 7 days (Table 1).
Fig. 1.
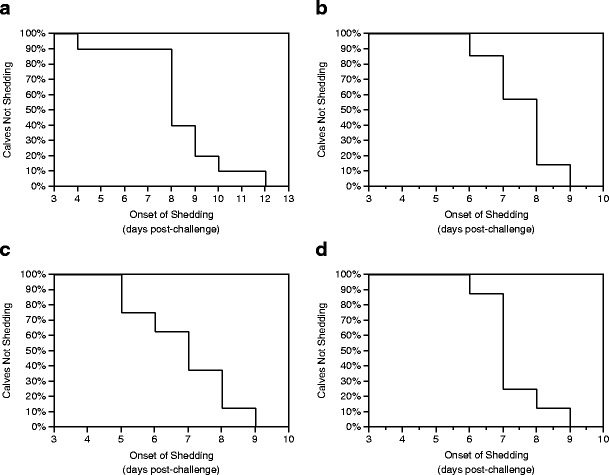
Probability of onset of fecal shedding post-challenge in calves dosed with 1 × 103 (a), 1 × 104 (b), 1 × 105 (c), or 1 × 106 (d) C. parvum oocysts
Table 1.
The average (mean ± SD), median, and range for the number of days post-challenge until the onset of fecal shedding, the number of days post-challenge until the cessation of fecal shedding, the number of days duration of fecal shedding post-challenge, and the log-total number of oocysts counted per gram of feces dry weight in calves experimentally challenged with 1 × 103 (group A), 1 × 104 (group B), 1 × 105 (group C), or 1 × 106 (group D) oocysts of C. parvum
| Group A | Group B | Group C | Group D | |
|---|---|---|---|---|
| Onset of fecal oocyst shedding (days) | ||||
| Mean | 9 ± 2 | 9 ± 1 | 8 ± 2 | 8 ± 1 |
| Median | 8 | 8 | 7 | 7 |
| Range | (4–12) | (6–9) | (5–9) | (6–9) |
| Cessation of fecal oocyst shedding (days) | ||||
| Mean | 18 ± 2 | 17 ± 1 | 17 ± 2 | 16 ± 1 |
| Median | 18.5 | 17 | 16.5 | 16 |
| Range | (14–21) | (15–19) | (14–21) | (13–16) |
| Duration of fecal oocyst shedding (days) | ||||
| Mean | 10 ± 2 | 9 ± 1 | 10 ± 2 | 8 ± 2 |
| Median | 10 | 9 | 10 | 9 |
| Range | (5–12) | (7–11) | (6–12) | (5–10) |
| Log-total oocysts counted per gram of feces dry weight | ||||
| Mean | 7.4 ± 0.3 | 8.0 ± 0.3 | 7.9 ± 0.5 | 7.7 ± 0.6 |
| Median | 7.6 | 7.9 | 8.1 | 7.7 |
| Range | (7.0–7.7) | (7.5–8.6) | (7.1–8.4) | (6.8–8.9) |
The earliest cessation of fecal shedding was at 13 days post-challenge in a calf dosed with 1 × 106 oocysts, and the latest cessation of fecal shedding was at 21 days post-challenge in two calves dosed with 1 × 103 and 1 × 105 oocysts. Similar to the onset of fecal shedding, among the four dose groups, the number of days until the cessation of fecal oocyst shedding post-challenge also tended to be influenced by dose magnitude (p = 0.08) (Table 1). The average duration of fecal oocyst shedding in days was 9.2 ± 1.8 (n = 33). There was not a difference in the duration of fecal oocyst shedding across the four dose groups (p = 0.23).
Figure 2 shows the distributions of the total amount of oocysts counted per gram of feces among the different dose magnitudes. Higher magnitudes of exposure exhibited more variability in the range of fecal oocyst shedding (Table 1). The log-total number of oocysts counted per gram of feces dry weight was different across the four dose groups (p = 0.04) (n = 36) (Fig. 2). As shown in Table 2, calves in group A (1 × 103), the lowest magnitude dosing group, tended to have the least amount of total oocysts counted per gram of feces dry weight among the four groups; and in post-hoc analysis, group A differed from group B (1 × 104) (p = 0.1), group C (1 × 105) (p = 0.09), and group D (1 × 106) (p = 0.15).
Fig. 2.
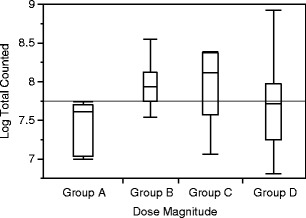
The total number of oocysts counted per gram of feces dry weight (log-transformed) by dose magnitude [1 × 103 (group A), 1 × 104 (group B), 1 × 105 (group C), or 1 × 106 (group D)] among calves that shed oocysts (n = 33) following experimental challenge with C. parvum
Table 2.
Regression analysis for the log-total amount of oocysts counted for all calves that shed C. parvum oocysts after oral challenge
| Factor | Regression coefficient | SE | P value |
|---|---|---|---|
| Intercept | 10.97 | 1.63 | <0.0001 |
| Oral exposure (number of oocysts log transformed) | −0.06 | 0.11 | 0.62 |
| Onset of fecal shedding (days post-challenge) | −0.28 | 0.09 | 0.004 |
| Serum total protein (g/dl) | −0.13 | 0.28 | 0.65 |
Given the difference in total amount of oocysts shed across the four dose groups, we wanted to examine the factors associated with this difference while controlling for serum total protein, the number of days until onset of fecal shedding, and the magnitude of oral exposure. Although the univariate results were suggestive that the log-total amount of oocysts shed is affected by the magnitude of oral exposure, this was not observed (p = 0.62) (Table 2) when controlling for serum total protein, the number of days until the onset of fecal shedding, and the magnitude of oral exposure. However, there was an inverse relationship between the log-total amount of oocysts counted per gram of feces dry weight and the number of days to the onset of fecal shedding (p = 0.005). The log-total amount of oocysts counted per gram of feces dry weight decreased by 0.28 as the number of days to onset of fecal shedding increased by one (Table 2).
The pattern of fecal shedding over time for all calves exposed to C. parvum oocysts, irrespective of dose, was curvilinear (Fig. 3). The log-total oocysts counted per gram of feces dry weight increased with age (p < 0.0001), reached a peak, and declined (p < 0.0001) (Table 3).
Fig. 3.
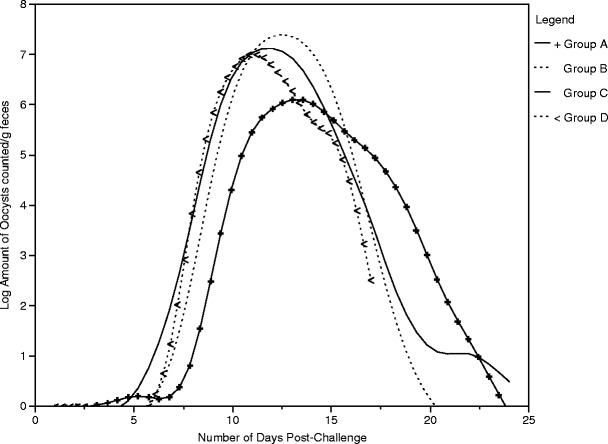
The pattern of fecal oocyst shedding over time for each dose group [1 × 103 (group A), 1 × 104 (group B), 1 × 105 (group C), or 1 × 106 (group D)] among calves that shed oocysts (n = 33) following experimental challenge with C. parvum
Table 3.
Regression analysis for the pattern of fecal shedding over time for all calves dosed with C. parvum oocysts regardless of dose magnitude
| Factor | Regression coefficient | SE | P value |
|---|---|---|---|
| Intercept | 2.2 | 0.21 | <0.0001 |
| Time | 0.11 | 0.001 | <0.0001 |
| (Time)2 | −0.01 | 0.0007 | <0.0001 |
Discussion
C. parvum infection is an important agricultural zoonosis (Fayer 2004). It causes severe illness in people, is associated with increased morbidity and mortality among calves, and can result in economic losses for dairy farms (Mor and Tzipori 2008; Waltner-Toews et al. 1986; Warnick et al. 1995). In the absence of a commercially available vaccine and consistently effective prophylaxis, the best means by which cryptosporidiosis can be controlled is through preventive measures. Previous studies in people and in calves in field settings suggest that smaller magnitudes of oral exposure reduce the duration of fecal oocyst shedding and may, in turn, reduce environmental loading, and thus reduce subsequent opportunities for exposure to the parasite (DuPont et al. 1995; Moore et al. 2003).
We have described the relationship between magnitude of oral exposure to C. parvum, and the onset, duration, and cessation of fecal shedding. We found a difference in the onset and cessation of fecal oocyst shedding, and in the log-total number of oocysts counted per gram of feces dry weight across four magnitudes of exposure, but we did not find a difference in the duration of fecal oocyst shedding. In contrast to our study, the study conducted by Moore et al. did find a significant difference in duration of fecal shedding with respect to size of inoculum in dairy calves (Moore et al. 2003). However, in that study, 75 calves were enrolled, and 29 calves died after day 3 of life. Among the eight that were necropsied, Salmonella spp, rotavirus, and coronavirus were recovered in addition to C. parvum. It is, therefore, possible that many of the calves enrolled in that study experienced a longer duration of shedding due to altered immune function associated with co-infection. In our study, all calves survived through to completion of the study. All calves were tested for infection with Salmonella spp, as well as for rotavirus and coronavirus infection, and all were negative, whereby eliminating enteric co-infection as a confounder. In addition to the study by Moore et al., another study conducted by DuPont et al. looking at the infectivity of C. parvum in people suggests that the size of oral inoculum not only influences the duration of fecal shedding but also influences the onset of fecal shedding (DuPont et al. 1995). However, people in this study with base-line antibody to the parasite experienced more severe clinical illness, which the authors attribute to increased susceptibility. This may have potentially confounded the onset and duration of fecal shedding. Both of these studies included oral inoculums that were less 1 × 103 oocysts in magnitude, whereas ours did not. Therefore, it is possible that the onset, cessation, and duration of fecal shedding are more appreciably impacted at lower dose magnitudes. It is also possible that the sample size for our study was not large enough to detect this difference.
The median time to onset of fecal shedding in our study was between 7 and 8 days across the four dose groups; the earliest onset was 4 days post-challenge, and the latest onset was 12 days post-challenge as is indicated in our survival analysis (Fig. 1). A paper by Moore et al. reports an overall mean time to onset of fecal shedding across all dose magnitudes of 7.4 days, which is similar to our reported median time. In our study, however, the mean time to onset of fecal shedding was slightly greater than in Moore’s study, with the two lowest dose groups having a mean of 9 days, and the two highest dose groups having a mean of 8 days.
With respect to fecal oocyst shedding across the four dose groups, three experimentally challenged calves never shed oocysts in their feces, one calf began shedding oocysts at 4 days post-challenge (Fig. 1), and another did not begin to shed oocysts until 12 days post-challenge (Fig. 1). Efforts were made to minimize extreme variability in parasite level effects by using the same field strain of oocysts that were at least 87 % viable. Likewise, efforts were also made to minimize extreme variability in calf level effects by enrolling calves from the same commercial dairy and ensuring adequate passive transfer of antibodies. These differences are best explained by normal variation in many factors acting in concert with one another including effects attributable to variations in calves, the parasite, and the environment. It should also be noted that among the three calves that did not shed and the two calves that shed earliest and latest in the study, all received a dose of 1 × 103 oocysts, which was the lowest dose magnitude we tested. It is conceivable that this was not a large enough infectious dose for the three calves that never shed, and the one calf that did not start to shed until day 12 post-challenge. Given the low infectious dose reported in people, it stands to reason that factors other than magnitude of infectious dose influenced fecal shedding for these calves (DuPont et al. 1995). These results indicate that the degree of fecal shedding of oocysts is influenced by multiple factors including dose magnitude, parasite lifecycle, parasite level effects, and calf level effects. It is also possible that different results might be attained with the use of a different strain, but without testing, we cannot be sure.
The results of this study show that calves exhibit the same pattern of fecal shedding over time regardless of dose magnitude. Calves that receive a lower oral dose begin shedding later and shed fewer total oocysts. This study also demonstrated that calves shed numbers of oocysts that greatly exceed the magnitude of the dose administered, which is partially attributable to the auto-infective stage of the parasite lifecycle in which sporozoites re-infect the epithelial cells of the intestinal lumen and thus initiate a new cycle of parasite development. While it was not evaluated in this study, it is possible that the auto-infective stage of the parasite life cycle may cause a delay in the onset of fecal shedding at lower infectious doses. Infected calves, in turn, void readily infective resistant oocysts into the surrounding environment. Given the heartiness of the parasite, the potential for large-scale contamination of the local environment and the low infectious dose, disease prevention remains to be the best control measure for cryptosporidiosis.
There is an indication that the onset of fecal shedding is influenced by the amount of oocysts to which calves are exposed; calves receiving lower doses of oocysts begin shedding later. Therefore, if onset of fecal oocyst shedding was delayed, environmental parasite loading could be reduced, as well as the risk of exposure for calves and people. Differences in fecal shedding varied across doses and between doses, indicating important effects at both the calf level and parasite level. Preventing calf exposure to the parasite entirely is not realistic in conventional calf-rearing systems where the pathogen is often endemic and therefore not an adequate means of disease prevention alone. Instead, an integrated approach to the prevention of cryptosporidiosis in calves should be undertaken in order to address parasite, calf, and environmental factors through the provision of clean and dry housing and an appropriate plane of nutrition. Future studies will be focused on the impact of delayed fecal shedding and reduced dose magnitudes on fecal oocyst shedding, as well as on delineating the dose–response relationship and determining the ID50 for fecal shedding in dairy calves.
Conclusion
Calves exhibit the same pattern of fecal shedding over time regardless of dose magnitude;
Across the four magnitudes of exposure (1 × 103, 1 × 104, 1 × 105, or 1 × 106), there is a difference in the onset and cessation of fecal oocyst shedding and in the log-total number of oocysts counted per gram of feces dry weight but no difference in the duration of fecal oocyst shedding;
The log-total amount of oocysts counted per gram of feces dry weight decreased as the number of days to onset of fecal shedding increased, i.e., fewer oocysts were shed by calves whose time to onset of fecal shedding was longer.
Acknowledgments
For their invaluable contribution, the authors thank the team of veterinary and pre-veterinary students who cared for the calves enrolled in the study, recorded data, and collected fecal samples.
Funding
Cornell University discretionary research support.
References
- Amadi B, Mwiya M, Musuku J, Watuka A, Sianongo S, Ayoub A, Kelly P. Effect of nitazoxanide on morbidity and mortality in Zambian children with cryptosporidiosis: a randomised controlled trial. Lancet. 2002;360:1375–1380. doi: 10.1016/S0140-6736(02)11401-2. [DOI] [PubMed] [Google Scholar]
- Anderson BC. Cryptosporidiosis in bovine and human health. J Dairy Sci. 1998;81:3036–3041. doi: 10.3168/jds.S0022-0302(98)75868-0. [DOI] [PubMed] [Google Scholar]
- Anderson VR, Curran MP. Nitazoxanide: a review of its use in the treatment of gastrointestinal infections. Drugs. 2007;67:1947–1967. doi: 10.2165/00003495-200767130-00015. [DOI] [PubMed] [Google Scholar]
- Anguish LJ, Ghiorse WC. Computer-assisted laser scanning and video microscopy for analysis of Cryptosporidium parvum oocysts in soil, sediment, and feces. Appl Environ Microbiol. 1997;63:724–733. doi: 10.1128/aem.63.2.724-733.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellosa ML, Nydam DV, Liotta JL, Zambriski JA, Linden TC, Bowman DD. A comparison of fecal percent dry matter and number of Cryptosporidium parvum oocysts shed to observational fecal consistency scoring in dairy calves. J Parasitol. 2011;97:349–351. doi: 10.1645/GE-2475.1. [DOI] [PubMed] [Google Scholar]
- Campbell AT, Robertson LJ, Smith HV. Viability of Cryptosporidium parvum oocysts: correlation of in vitro excystation with inclusion or exclusion of fluorogenic vital dyes. Appl Environ Microbiol. 1992;58:3488–3493. doi: 10.1128/aem.58.11.3488-3493.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappell CL, Okhuysen PC, Langer-Curry R, Widmer G, Akiyoshi DE, Tanriverdi S, Tzipori S. Cryptosporidium hominis: experimental challenge of healthy adults. AmJTrop Med Hyg. 2006;75:851–857. [PubMed] [Google Scholar]
- de Graaf DC, Vanopdenbosch E, Ortega-Mora LM, Abbassi H, Peeters JE. A review of the importance of cryptosporidiosis in farm animals. Int J Parasitol. 1999;29:1269–1287. doi: 10.1016/S0020-7519(99)00076-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuPont HL, Chappell CL, Sterling CR, Okhuysen PC, Rose JB, Jakubowski W. The infectivity of Cryptosporidium parvum in healthy volunteers. N Engl J Med. 1995;332:855–859. doi: 10.1056/NEJM199503303321304. [DOI] [PubMed] [Google Scholar]
- Fayer R, Santin M, Trout JM. Prevalence of Cryptosporidium species and genotypes in mature dairy cattle on farms in eastern United States compared with younger cattle from the same locations. Vet Parasitol. 2007;145:260–266. doi: 10.1016/j.vetpar.2006.12.009. [DOI] [PubMed] [Google Scholar]
- Fayer R. Cryptosporidium: a water-borne zoonotic parasite. Vet Parasitol. 2004;126:37–56. doi: 10.1016/j.vetpar.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Feltus DC, Giddings CW, Schneck BL, Monson T, Warshauer D, McEvoy JM. Evidence supporting zoonotic transmission of Cryptosporidium spp. in Wisconsin. J Clin Microbiol. 2006;44:4303–4308. doi: 10.1128/JCM.01067-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graczyk Z, Chomicz L, Kozlowska M, Kazimierczuk Z, Graczyk TK. Novel and promising compounds to treat Cryptosporidium parvum infections. Parasitol Res. 2011;109:591–594. doi: 10.1007/s00436-011-2290-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harp JA, Goff JP. Strategies for the control of Cryptosporidium parvum infection in calves. J Dairy Sci. 1998;81:289–294. doi: 10.3168/jds.S0022-0302(98)75578-X. [DOI] [PubMed] [Google Scholar]
- Jarvie BD, Trotz-Williams LA, McKnight DR, Leslie KE, Wallace MM, Todd CG, Sharpe PH, Peregrine AS. Effect of halofuginone lactate on the occurrence of Cryptosporidium parvum and growth of neonatal dairy calves. J Dairy Sci. 2005;88:1801–1806. doi: 10.3168/jds.S0022-0302(05)72854-X. [DOI] [PubMed] [Google Scholar]
- Jenkins MB, Anguish LJ, Bowman DD, Walker MJ, Ghiorse WC. Assessment of a dye permeability assay for determination of inactivation rates of. Appl Environ Microbiol. 1997;63:3844–3850. doi: 10.1128/aem.63.10.3844-3850.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins MB, Walker MJ, Bowman DD, Anthony LC, Ghiorse WC. Use of a sentinel system for field measurements of Cryptosporidium parvum oocyst inactivation in soil and animal waste. Appl Environ Microbiol. 1999;65:1998–2005. doi: 10.1128/aem.65.5.1998-2005.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J, Alderisio KA, Xiao L. Distribution of Cryptosporidium genotypes in storm event water samples from three watersheds in New York. Appl Environ Microbiol. 2005;71:4446–4454. doi: 10.1128/AEM.71.8.4446-4454.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53(282):457–481. doi: 10.1080/01621459.1958.10501452. [DOI] [Google Scholar]
- Lefay D, Naciri M, Poirier P, Chermette R. Efficacy of halofuginone lactate in the prevention of cryptosporidiosis in suckling calves. Vet Rec. 2001;148:108–112. doi: 10.1136/vr.148.4.108. [DOI] [PubMed] [Google Scholar]
- Lindsay DS, Upton SJ, Owens DS, Morgan UM, Mead JR, Blagburn BL. Cryptosporidium andersoni n. sp. (Apicomplexa: Cryptosporiidae) from cattle, Bos taurus. J Eukaryot Microbiol. 2000;47:91–95. doi: 10.1111/j.1550-7408.2000.tb00016.x. [DOI] [PubMed] [Google Scholar]
- MacKenzie WR, Schell WL, Blair KA, Addiss DG, Peterson DE, Hoxie NJ, Kazmierczak JJ, Davis JP. Massive outbreak of waterborne Cryptosporidium infection in Milwaukee, Wisconsin: recurrence of illness and risk of secondary transmission. Clin Infect Dis. 1995;21:57–62. doi: 10.1093/clinids/21.1.57. [DOI] [PubMed] [Google Scholar]
- Mead JR. Cryptosporidiosis and the challenges of chemotherapy. Drug Resist Updat. 2002;5:47–57. doi: 10.1016/S1368-7646(02)00011-0. [DOI] [PubMed] [Google Scholar]
- Moore DA, Atwill ER, Kirk JH, Brahmbhatt D, Herrera Alonso L, Hou L, Singer MD, Miller TD. Prophylactic use of decoquinate for infections with Cryptosporidium parvum in experimentally challenged neonatal calves. J Am Vet Med Assoc. 2003;223:839–845. doi: 10.2460/javma.2003.223.839. [DOI] [PubMed] [Google Scholar]
- Mor SM, Tzipori S. Cryptosporidiosis in children in Sub-Saharan Africa: a lingering challenge. Clin Infect Dis. 2008;47:915–921. doi: 10.1086/591539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan-Ryan UM, Fall A, Ward LA, Hijjawi N, Sulaiman I, Fayer R, Thompson RC, Olson M, Lal A, Xiao L. Cryptosporidium hominis n. sp. (Apicomplexa: Cryptosporidiidae) from Homo sapiens. J Eukaryot Microbiol. 2002;49:433–440. doi: 10.1111/j.1550-7408.2002.tb00224.x. [DOI] [PubMed] [Google Scholar]
- Nydam DV, Wade SE, Schaaf SL, Mohammed HO. Number of Cryptosporidium parvum oocysts or Giardia spp cysts shed by dairy calves after natural infection. Am J Vet Res. 2001;62:1612–1615. doi: 10.2460/ajvr.2001.62.1612. [DOI] [PubMed] [Google Scholar]
- O’Handley RM, Cockwill C, McAllister TA, Jelinski M, Morck DW, Olson ME. Duration of naturally acquired giardiosis and cryptosporidiosis in dairy calves and their association with diarrhea. J Am Vet Med Assoc. 1999;214:391–396. [PubMed] [Google Scholar]
- Ollivett TL, Nydam DV, Bowman DD, Zambriski JA, Bellosa ML, Linden TC, Divers TJ. Effect of nitazoxanide on cryptosporidiosis in experimentally infected neonatal dairy calves. J Dairy Sci. 2009;92:1643–1648. doi: 10.3168/jds.2008-1474. [DOI] [PubMed] [Google Scholar]
- Robinson G, Thomas AL, Daniel RG, Hadfield SJ, Elwin K, Chalmers RM. Sample prevalence and molecular characterisation of Cryptosporidium andersoni within a dairy herd in the United Kingdom. Vet Parasitol. 2006;142:163–167. doi: 10.1016/j.vetpar.2006.06.031. [DOI] [PubMed] [Google Scholar]
- Santin M, Trout JM, Xiao L, Zhou L, Greiner E, Fayer R. Prevalence and age-related variation of Cryptosporidium species and genotypes in dairy calves. Vet Parasitol. 2004;122:103–117. doi: 10.1016/j.vetpar.2004.03.020. [DOI] [PubMed] [Google Scholar]
- SAS Institute Inc. (1989–2007) JMP. 7.0
- Trotz-Williams LA, Martin DS, Gatei W, Cama V, Peregrine AS, Martin SW, Nydam DV, Jamieson F, Xiao L. Genotype and subtype analyses of Cryptosporidium isolates from dairy calves and humans in Ontario. Parasitol Res. 2006;99:346–352. doi: 10.1007/s00436-006-0157-4. [DOI] [PubMed] [Google Scholar]
- Trotz-Williams LA, Wayne Martin S, Leslie KE, Duffield T, Nydam DV, Peregrine AS. Calf level risk factors for neonatal diarrhea and shedding of Cryptosporidium parvum in Ontario dairy calves. Prev Vet Med. 2007;82:12–28. doi: 10.1016/j.prevetmed.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waltner-Toews D, Martin SW, Meek AH. The effect of early calfhood health status on survivorship and age at first calving. Can J Vet Res. 1986;50:314–317. [PMC free article] [PubMed] [Google Scholar]
- Warnick LD, Erb HN, White ME. Lack of association between calf morbidity and subsequent first lactation milk production in 25 New York Holstein herds. J Dairy Sci. 1995;78:2819–2830. doi: 10.3168/jds.S0022-0302(95)76912-0. [DOI] [PubMed] [Google Scholar]
- Xiao L, Feng Y. Zoonotic cryptosporidiosis. FEMS Immunol Med Microbiol. 2008;52:309–323. doi: 10.1111/j.1574-695X.2008.00377.x. [DOI] [PubMed] [Google Scholar]
- Xiao L, Herd RP. Infection pattern of Cryptosporidium and Giardia in calves. Vet Parasitol. 1994;55:257–262. doi: 10.1016/0304-4017(93)00645-F. [DOI] [PubMed] [Google Scholar]
- Xiao L, Herd RP. Quantitation of Giardia cysts and Cryptosporidium oocysts in fecal samples by direct immunofluorescence assay. J Clin Microbiol. 1993;31:2944–2946. doi: 10.1128/jcm.31.11.2944-2946.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]


