Fig. 5.
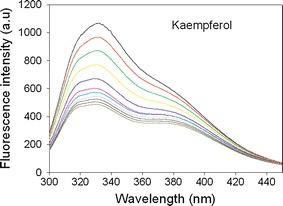
The fluorescence quenching of SaPriA by kaempferol. Compound concentrations, from the top down, are 0 to 10 μM. Fluorescence titration was performed using a spectrofluorimeter (Hitachi F-2700). An aliquot amount of kaempferol was added into the solution containing SaPriA (0.5 μM), 20 mM HEPES, and 100 mM NaCl at pH 7.0. The K d value was obtained by the equation: ΔF = ΔFmax − K d(ΔF/[compound]) (Enzyme Kinetics module of Sigma-Plot)
