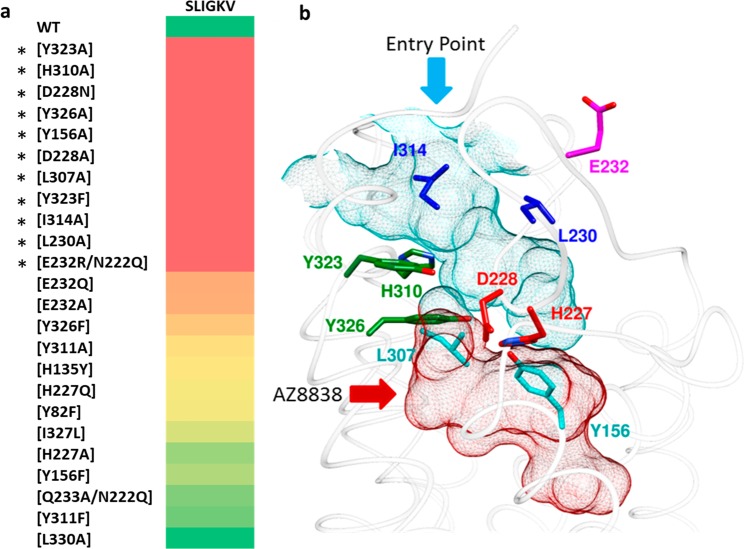
Figure 1.
Identification of the orthosteric binding site. (a) Heat plot of fold change of SLIGKV-induced activation of calcium mobilization at mutant PAR2 receptors presented on a color scale where red and green correspond to >10-fold drop in potency and effect similar to that of WT, respectively. Numerical data are presented in Table 1. Asterisks highlight the residues identified as important for SLIGKV-induced activation of PAR2. (b) Crystal structure of PAR2 (PDB ID: 5NDD)16 with side chains of key residues shown as sticks. The predicted orthosteric binding site is depicted as a surface mesh, encompassing an entry point from the extracellular space (cyan) and the AZ8838 binding site (red). Residues have been color coordinated based on their spatial arrangement.