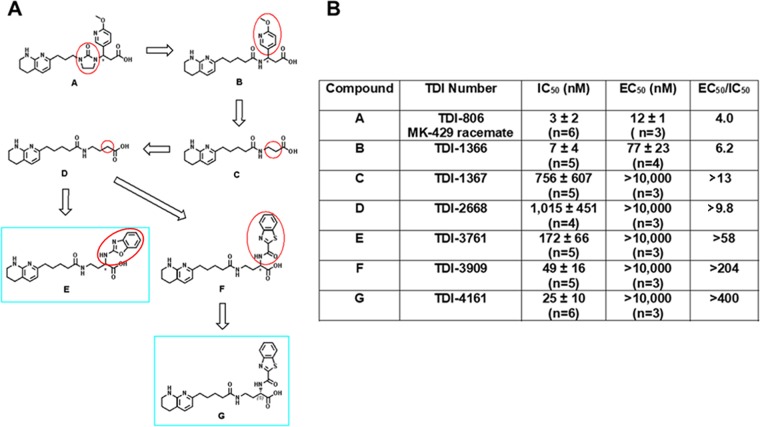
Figure 2.
Development of TDI-4161 and TDI-3761. (A) Structures. Structural modifications began with TDI-806, the racemate of MK-429 (A). The first step involved removing the imidazolinone ring, yielding TDI-1366 (B). This compound was further simplified by removing the aromatic side chain, yielding TDI-1367 (C). Increasing the length of TDI-1367 by one carbon resulted in TDI-2668 (D). The racemic compounds TDI-3761 (E) and TDI-3909 (F) were produced by adding aromatic groups in the α position of the TDI-2668 carboxylic acid. Both the R and S enantiomers of TDI-3761 had properties similar to those of TDI-3761 (see text for values), whereas TDI-4161 (G), the S enantiomer of TDI-3909 was more potent and equally selective when compared to TDI-4169, the R enantiomer (not shown). (B) Characteristics of compounds.