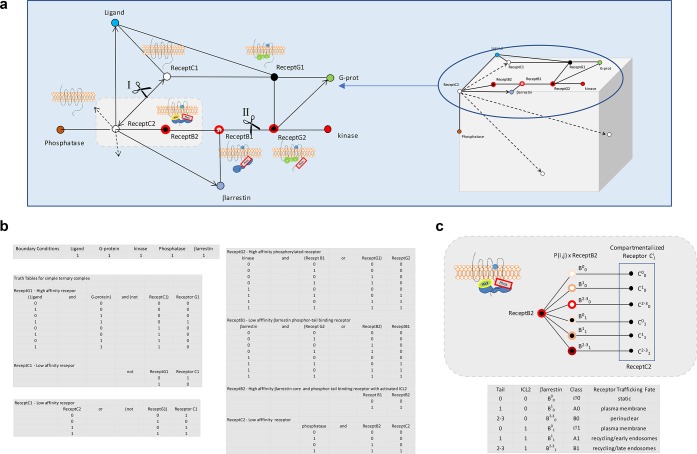
Figure 8.
Boolean network analysis of β-arrestin-receptor interactions for determination of receptor trafficking fate. This type of mesh analysis in which the state of a protein changes according to logical rules and the state of precursors provides a simplification in modeling biological processes in comparison to more demanding mathematically rigorous methods. Mesh nodes represent components participating in the biochemical process and edges connecting nodes the processes. Cartoons of the different receptor complexes corresponding to distinct nodes are shown juxtaposed to corresponding nodes. (a) The leftmost network represents the transition of inactive plasma membrane receptor C1 through multiple stages to C2 that are driven by interactions with ligand, G-protein, β-arrestin, and phosphatase. The upper network between cuts at positions I and II delineate the plasma membrane receptor fates C1 through the transition to GRK-phosphorylated receptor G2 residing in a lipid-raft signaling compartment. The lower half of the cut network represents what happens to the receptor subsequent to interaction with β-arrestin. The fate of receptor C3 is determined by the affinity of receptor B2 for β-arrestin and by the compartment in which β-arrestin dissociation from the receptor occurs. The cube on the right with its multiple faces represents fates where trafficking has occurred to compartments other than the plasma membrane. (b) Boolean logic table describing the relationships occurring at each receptor node. (c) Probability based fate determinations, P(i, j), for trafficking receptors Cij resulting from affinity and dissociation differences of β-arrestin from receptor B2. The table below the cartoon shows six trafficking fates for ligand bound receptors due to the differential binding occurring at ICL2 (0 and 1) or the tail (0, 1, 2–3). Conventional class A and B receptors are now designated in the table by A1 and B1, and the corresponding β-arrestin affinity for the ligand bound receptor due to the ICL2 and the C-tail is indicated by the superscript and subscripts of β-arrestin as Bij. The new class designation Ø corresponds to an absent to very weak ICL2 core interaction permissive for a β-arrestin dissociating receptor remaining at the plasma membrane.