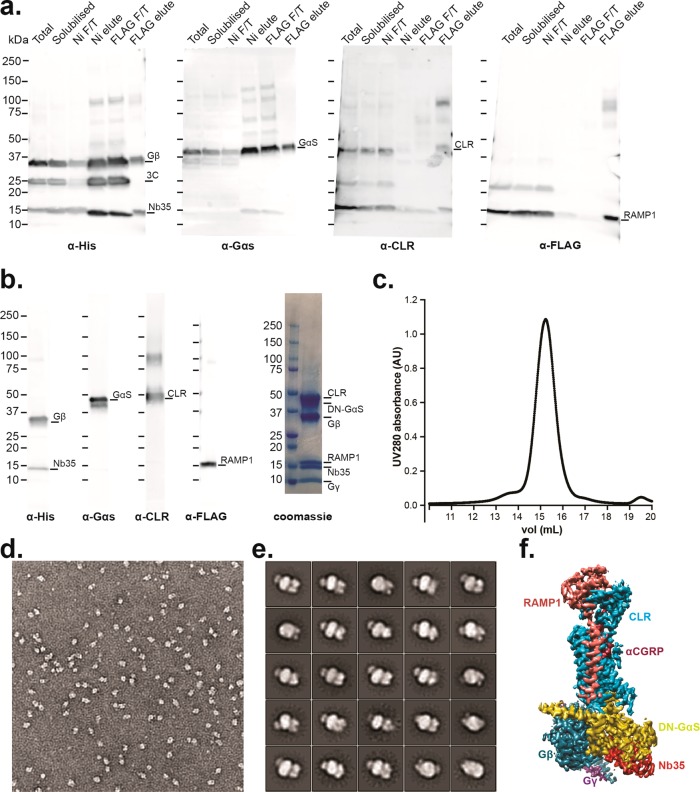
Figure 4.
DN-Gαs allows purification and structure determination of the fully active heteromeric CGRP receptor. (a) Western blots of purification fractions of the CGRP active complex using WT-Gαs showing purification is possible but yield is poor (see text): (left) anti-His antibody against His tags on Gβ1 and Nb-35; (middle left) anti-Gαs antibody for Gαs detection; (middle right) anti-CLR antibody for CLR detection; (right) anti-FLAG antibody against the FLAG tag on RAMP1. (b) (Left) Western blot of the final purified fraction of the CGPR complex formed using DN-Gαs showing that all components are present; (right) coomassie stained gel showing stoichiometric recovery of proteins. (c) Monodisperse peak of the purified complex following size exclusion chromatography. (d) Negative stain EM micrograph. (e) 2D class averages from the negative stain EM data. (f) 3D cryo-EM map from single particle cryo-EM determination of structure, colored according to each protein subunit (the 3D map was adapted from Liang et al.19).