Table 1.
Non-prenylated flavonoid aglycones isolated from P. tomentosa
| Name of compound and ID | Isolation source | Biological activity | Chemical structure | ||||
|---|---|---|---|---|---|---|---|
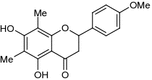
| Matteucinol (syn. 4′-O-methylfarrerol) (1) (2S) | Leaves (Zhu et al. 1986) | Weak cytotoxic activity in vitro against human leukaemia (HL-60) and human hepatoma (SMMC-7721) cell lines (Zhao et al. 2012); no α-glucosidase inhibitory effect (Zhao et al. 2009); weak aldose-reductase inhibitory activity (Kadota et al. 1994); moderate inhibitory effect on human immunodeficiency virus-1 protease (Lee et al. 2008); no activity against lipopolysaccharide (LPS)-induced NO production in RAW 264.7 macrophages (Li et al. 2011) |
|
||||
|
|||||||
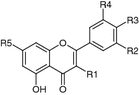
| R1 | R2 | R3 | R4 | R5 | |||
|---|---|---|---|---|---|---|---|
| Apigenin (2) | Leaves (Zhao et al. 2012), flowers (Chen et al. 2009; Jiang et al. 2004) | Antioxidant (Prince Vijeya Singh et al. 2004); antibacterial, anti-inflammatory, antispasmodic, antidiarrhoic, antiproliferative, vasorelaxant (Jiang et al. 2004); neuroprotective (Losi et al. 2004); cardioprotective (Psotová et al. 2004); chemopreventive activity against skin cancer (Chen et al. 2009); activity reviewed by Shukla and Gupta (2009) and Patel et al. (2007) | H | H | OH | H | OH |
| Kaempferol (3) | Leaves (Si et al. 2008a) | Cytotoxic, pro-apoptic (suppresses cell metastasis via inhibition of the ERK-p38-JNK and AP-1 signaling pathways in U-2 OS human osteosarcoma cells) (Chen et al. 2013); antioxidant (e.g., attenuates bladder hyperactivity caused by potassium chloride after protamine sulphate-induced bladder injury) (Huang et al. 2014); impact on human health and cancer chemoprevention reviewed by Chen and Chen (2013), and Calderon-Montano et al. (2011) | OH | H | OH | H | OH |
| Luteolin (4) | Leaves (Si et al. 2008a; Zhang and Li 2011) | Memory-improving (Yoo et al. 2013); inhibition of α-amylase activity (Funke and Melzig 2005); cytotoxic (BGC-823 gastric carcinoma xenografts in nude mice) (Lu et al. 2013); vascular protective (Si et al. 2013); biological activity reviewed by Lopez-Lazaro (2009) | H | OH | OH | H | OH |
| Quercetin (5) | Bark (Si et al. 2011b), leaves (Si et al. 2008a) | Antifibrotic (Yoon et al. 2012); antioxidant, antiproliferative, anti-inflammatory, cardioprotective (Chen et al. 2013); neuroprotective (Ghosh et al. 2013); anti-diabetic [dose-dependent inhibition of both Na+/K+ ATPase and sodium hydrogen exchanger in type 2 diabetic erythrocytes (Mishra and Rizvi 2012); inhibition of PI3K (Koch et al. 2013)]; antiviral [anti HCV (Khachatoorian et al. 2012); HCMV (Cotin et al. 2012)]; no anti-HIV activity in vitro tested on H9 cells in the absence of toxicity (Tang et al. 1994); review of bioactivities by Russo et al. (2012) | OH | H | OH | OH | OH |
| 7,3′-Dimethylquercetin (syn. rhamnazin) (6) | Leaves, green immature fruit (Wollenweber et al. 2008) | Antimicrobial activity, poor antifungal effect (Omosa et al. 2014); low antimicrobial activity, low toxicity against human lymphocytes and monocytes, antioxidant/anti-inflammatory activity (Martini et al. 2004; Pelzer et al. 1998); low affinity to acetylcholinesterase (Remya et al. 2012); low inhibitory effect on NO production in RAW264.7 cells (Sudsai et al. 2013); no activity against HSV-1, low toxicity against Vero cells (Tian et al. 2009); no antioxidant activity (Takamatsu et al. 2003); no trypanocidal activity (Grael et al. 2000); activity against lipid peroxidation in rat liver microsomes (Yun et al. 2000); cytotoxicity against TK-10, MCF-7 and UACC-62 cells (Lopez-Lazaro et al. 2000); no inhibition of glycolysis in various tumor cells (Suolinna et al. 1975) | OH | OMe | OH | H | OMe |
| 7,3′,4′-Trimethylquercetin (7) | Thrombin inhibition (Shi et al. 2012); inhibition of IL-4 synthesis in basophils (Hirano et al. 2006); weak trypanocidal activity (Jordao et al. 2004); weak inhibition of NO production in LPS-activated mouse peritoneal macrophages (Matsuda et al. 2003); weak inhibition of degranulation of RBL-2H3 cells (Mastuda et al. 2002); inhibition of Pgp activity (Scambia et al. 1994); no inhibition of glycolysis in a variety of tumor cells (Suolinna et al. 1975) | OH | OMe | OMe | H | OMe | |
| 7,3′,4′-Trimethylmyricetin (8) | Weak inhibition of NO production in LPS-activated mouse peritoneal macrophages (Matsuda et al. 2003); weak inhibition of degranulation of RBL-2H3 cells (Mastuda et al. 2002) | OH | OMe | OMe | OH | OMe | |
| 7,3′,4′,5′-Tetramethylmyricetin (9) | OH | OMe | OMe | OMe | OMe | ||
|
|||||||
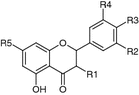
| R1 | R2 | R3 | R4 | R5 | |||
|---|---|---|---|---|---|---|---|
| (+)-Catechin (10) (2R, 3S) | Leaves (Si et al. 2008a) | Antiviral (HCV) (Khachatoorian et al. 2012); antimicrobial (Mankovskaia et al. 2013); antioxidant, e.g., adjunctive therapy for the treatment of Parkinson’s disease (Teixeira et al. 2013); osteogenic (Wei et al. 2011); anti-proliferative, anti-inflammatory, antifibrotic (GRX cells) (Bragança de Moraes et al. 2012); activity reviewed, for example, by Bansal et al. (2013) | OH | H | OH | OH | OH |
| Dihydrotricin (11) (2S) | Fruits (Šmejkal et al. 2007a) | Antioxidant, vasorelaxant, low anti-platelet aggregation activity (Chang et al. 2010) | H | OMe | OH | OMe | OH |
| (−)-Epicatechin (12) (2R, 3R) | Leaves (Si et al. 2008a) | Antifungal (Betts et al. 2013); improves muscle mitochondrial structure (Taub et al. 2012); antioxidant, cardioprotective (Paneerselvam et al. 2010); cytotoxic (HT29 human colon adenocarcinoma cells) (Sánchez-Tena et al. 2013); anti-inflammatory (Fu et al. 2013); activity reviewed, for example, by Fraga and Oteiza (2011) | OH | H | OH | OH | OH |
| Homoeriodictyol (syn. hesperetin) (13) (2S) | Leaves (Zhang and Li 2011) | Major flavonoid of citrus, activity reviewed by Khan and Zill-E-Huma (2014) | H | H | OH | OMe | OH |
| Naringenin (14) (2S) | Bark (Si et al. 2011b), leaves (Si et al. 2008a) | Anti-diabetic (inhibition of PI3K) (Koch et al. 2013); antiviral [anti HCV (Khachatoorian et al. 2012), HCMV (Cotin et al. 2012)]; antimicrobial (de Aguiar et al. 2013); anti-inflammatory (Jayaraman et al. 2012); antioxidant (Wang et al. 2012); cytotoxic (ERα-transfected HeLA cells, ERα-containing HepG2 cells) (Bulzomi et al. 2010); no antiparasitic activity against Leishmania spp. (major, chagasi, braziliensis, amazonensis), Trypanosoma cruzi (Grecco Sdos et al. 2012); major flavonoid of citrus, activity reviewed by Khan and Zill-E-Huma (2014) | H | H | OH | H | OH |
| Taxifolin (15) (2R, 3R) | Leaves (Si et al. 2008a) | Inhibition of β-amyloid aggregation (Sato et al. 2013); potential for atopic dermatitis treatment (blocks hERG K+ channels) (Yun et al. 2013); cytotoxic, pro-apoptotic (DU145 human prostate carcinoma cells) (Zhang and Coultas 2013); hypotensive (ACEI), anti-inflammatory, antioxidant (diminished 5-lipoxygenase and NADPH oxidase activity) (Arutyunyan et al. 2012); activity reviewed by Weidmann (2012) | OH | OH | OH | H | OH |
| 5,4′-Dihydroxy-7,3′-dimethoxyflavanone (16) (2S) | Flowers (Chen et al. 2009; Jiang et al. 2004; Kim et al. 2010a, b) | Inactive against glutamate-induced neurotoxicity studied in primary-cultured rat cortical cells (Kim et al. 2010a, b); no trypanocidal activity (Grael et al. 2000) | H | H | OH | OMe | OMe |
| 5-Hydroxy-7,3′,4′-trimethoxyflavanone (17) (2S) | Flowers (Kim et al. 2010a, b) | Inactive against glutamate-induced neurotoxicity studied in primary-cultured rat cortical cells (Kim et al. 2010a, b); inhibition of inflammation by induction of synovial apoptosis of fibroblast-like synoviocytes through caspase 3 activation in rats with adjuvant arthritis (Li et al. 2010, 2013); inhibition of JAK2-STAT3 signal pathway in rats (Li et al. 2012); inhibition of phosphodiesterase 4 (Yang et al. 2011); low toxicity on B16F10 and SK-MEL-1 melanoma cells (Rodriguez et al. 2002); antimutagenic activity (Miyazawa et al. 2000) | H | H | OMe | OMe | OMe |
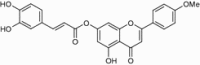
| 7-Caffeoyl-acacetin (syn. 7-caffeoyl-4′-methoxyapigenin) (18) | Stem bark (Si et al. 2009) |
|
|||||
