Table 4.
Phenolic glycosides isolated from P. tomentosa
| Name of compound and ID | Isolation | Biological activity | Chemical structure |
|---|---|---|---|
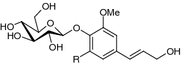
|
| R | |||
|---|---|---|---|
| Coniferin (syn. abietin, coniferosid, laricin) (69) | Bark (Sticher and Lahloub 1982; Zhu et al. 1986) | Antiphlogistic (Choi et al. 2004), low COX-2/1 and 5-LOX inhibition (Wang et al. 2013; Diaz Lanza et al. 2001); low antioxidant activity in vitro (DPPH) (She et al. 2013); stimulation osteoblastic bone formation in vitro (Ding et al. 2010); concentration-dependent contractions in rat aortic rings (Deliorman et al. 2000); inhibition of ADP-induced platelet aggregation (Panossian et al. 1998); hypotensive in rats (Matsubara et al. 1991) | H |
| Syringin (syn. eleutherosid B) (70) | Bark (Sticher and Lahloub 1982; Zhu et al. 1986) | Antidepressant (Kurkin et al. 2006), antiphlogistic, antinociceptive (Choi et al. 2004); peroxyl radical scavenging capacity (Kim et al. 2010a, b), inhibition of NO production in LPS-induced RAW 264.7 cells (Lee et al. 2009); enhances memory in aged rats (Huang et al. 2013a, b); protective effects against Aβ(25–35)-induced atrophies of axons and dendrites (Bai et al. 2011; Tohda et al. 2008); immunomodulatory activity in PMN phagocytic function test (Sharma et al. 2012); hepatoprotective in mice (Gong et al. 2014); adiponectin receptor 2 agonist (Sun et al. 2013); low COX and 5-LOX inhibition (Diaz Lanza et al. 2001); concentration-dependent contractions in rat aortic rings (Deliorman et al. 2000); inhibition of ADP-induced platelet aggregation (Panossian et al. 1998; Iizuka et al. 2005); moderate in vitro cytotoxic activities against A549 and HL-60 (Yang et al. 2012); antitumour activity in sarcoma S180 transplanted mice (Zhang et al. 2007); weak inhibition of snake PDE I (Chai et al. 2009); hypoglycemic effect (Liu et al. 2008; Niu et al. 2008a, b; Niu et al. 2007); adaptogenic activity reviewed by Panossian and Wagner (2005) | OMe |
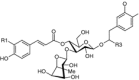
|
| R1 | R2 | R3 | |||
|---|---|---|---|---|---|
| Acteoside (syn. verbascoside) (71) | Bark (Si et al. 2011b; Sticher and Lahloub 1982; Zhu et al. 1986), wood (Si et al. 2008d), sapwood (Ota et al. 1993), fruit (Šmejkal et al. 2007b), leaves (Schilling et al. 1982), young plant (Damtoft and Jensen 1993) | Antioxidant, anti-inflammatory (Lin et al. 2006; Šmejkal et al. 2007b); antihepatotoxic (Xiong et al. 1998), antimicrobial (Kurkin 2003), antiviral (Kim et al. 2001); immuno-modulatory (Akbay et al. 2002); antihypertensive (Ahmad and Rizwani 1995); neuroprotective (Koo et al. 2005); cytotoxic (e.g., human erythro-leukemia cell line K562 (Šmejkal et al. 2008a), antiestrogen in breast cancer cells MCF7 and osteoblast without any effect on endometrial cancer cells Ishikawa (Papoutsi et al. 2006), bioactivities reviewed by He et al. (2011) | OH | OH | H |
| β-Oxoacteoside (syn. tomentoside A) (72) | Antioxidant (Tozuka et al. 2005) | OH | OH | =O | |
| Martynoside (73) | Stem (Kang et al. 1994) | Antioxidant (Jimenéz and Riguera 1994); antihypertensive (Kang et al. 2003); cytotoxic (antiestrogen in breast cancer cells MCF7 via the ER-pathway, osteoblasts KS483, endometrial cancer cells Ishikawa) (Papoutsi et al. 2006) | OMe | OMe | H |
| Campneoside I (74) | Wood (Si et al. 2008d), stem (Kang et al. 1994) | Antibacterial against Staphylococcus and Streptococcus species (MIC 150 μg/ml) (Kang et al. 1994) | OH | OH | OMe |
| Campneoside II (syn. β-hydroxy-acteoside) (75) | Bark (Si et al. 2011b), wood (Si et al. 2008b) | Antibacterial, anti-inflammatory (Jimenéz and Riguera 1994), anticomplement (Si et al. 2008b) | OH | OH | OH |
| Ilicifolioside A (76) | wood (Si et al. 2008b) | Anticomplement (Si et al. 2008b) | OH | OH | OEt |
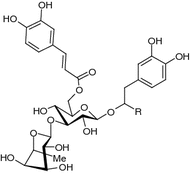
|
|||||
| R | |||
|---|---|---|---|
| Isoacteoside (syn. isoverbascoside) (77) | Bark (Si et al. 2011b), wood (Si et al. 2008d), sapwood (Ota et al. 1993), fruit (Šmejkal et al. 2007b), leaves (Schilling et al. 1982), young plant (Damtoft and Jensen 1993) | Antioxidant (Šmejkal et al. 2007b), xanthine oxidase inhibition (Kong et al. 1999); suppression of glutamate induced neurotoxicity (Koo et al. 2005); antioxidant, immunosuppressive (Jimenéz and Riguera 1994); cytotoxic (e.g., human erythro-leukemia cell line K562) (Šmejkal et al. 2008a) | H |
| Isocampneoside I (78) | Wood (mixture of R- and S-epimers) (Si et al. 2008d) | Inhibition of D-galactosamine induced cytotoxicity in primary cultured mouse hepatocytes (Pan et al. 2010) | OMe |
| Isocampneoside II (79) | Bark (Si et al. 2011b), wood (Si et al. 2008b) | Aldose reductase inhibitor (potential against diabetic complications) (Kim et al. 2011); anticomplement (Si et al. 2008b); antioxidant, neuro-protective on hydrogen peroxide-induced oxidative injury in PC12 cells (Si et al. 2013). | OH |
| Isoilicifolioside A (80) | Wood (Si et al. 2008b) | Anticomplement (Si et al. 2008b) | OEt |
| Cistanoside F (81) | Bark (Si et al. 2011b) | Antioxidant (Xiong et al. 1996); vasorelaxant (Yoshikawa et al. 2006); interaction with bovine serum albumin (Wu et al. 2012) | 
|
