Fig. 3.
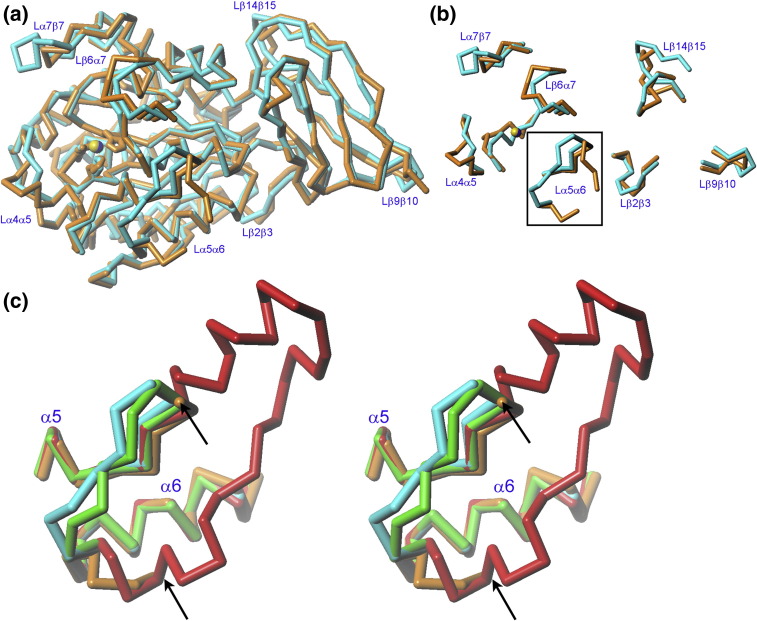
Structural comparison of DmCPD1Bs. (a) Superimposition of the Cα traces of DmCPD1Bs (orange; yellow zinc ion) and duck CDP domain II (PDB 1QMU; cyan; blue zinc ion). (b) Detail of (a) showing only those loop segments that evince major conformational differences between the two structures. The regulatory loop (Lα5α6) is framed. (c) Superimposition of the Cα traces of DmCPD1Bs (orange), duck CDP domain II (PDB 1QMU; cyan21, 48), human CPM (PDB 1UWY; green40), and human CPN (PDB 2NSM; red41) corresponding to segments equivalent to α5, Lα5α6, and α6 of the fruit fly enzyme. Orientation as in (a). The arrows point to the residues that flank the flexible region of the regulatory loop in DmCPD1Bs: up to nine residues are missing in each of the protomers.