The file Dataset-Fig1.xlsx contains:
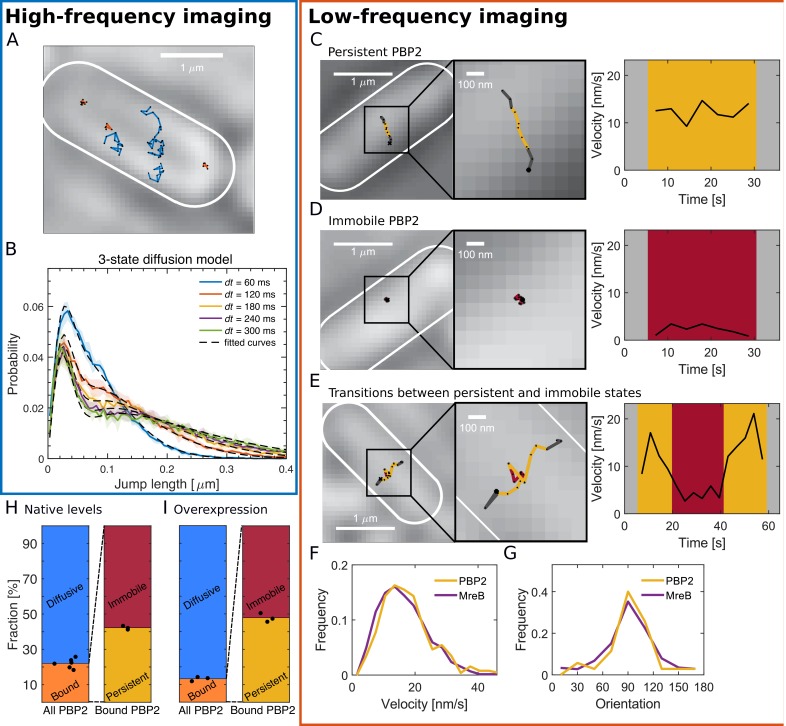
Figure 1H-I Number of tracks, average diffusion constants, bound and persistent fractions for PAmCherry-PBP2 for native and overexpression conditions, including p-values for bound and persistent fractions.
Figure 1F-G Numerical values of the distribution of speeds and orientations of PAmCherry-PBP2 and MreB-msfGFP tracks from low frequency imaging and number of tracks considered.
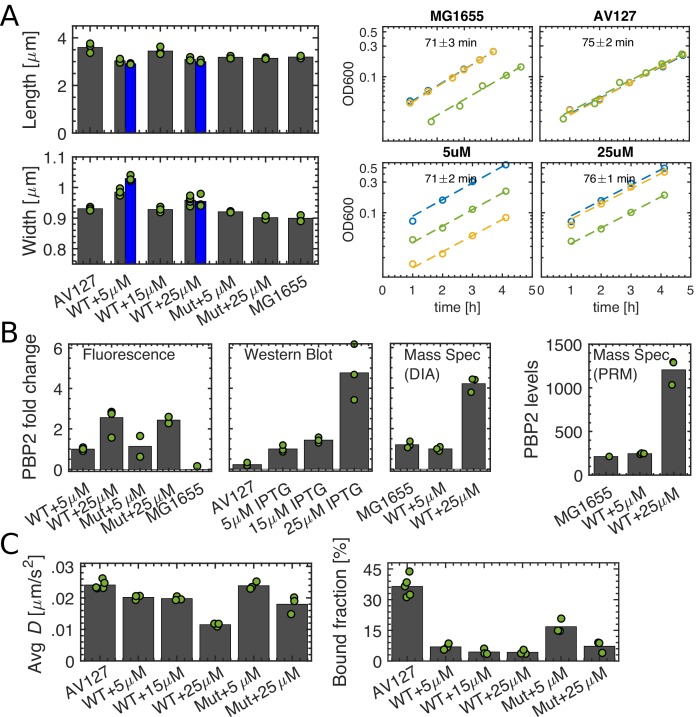
Figure 1—figure supplement 1B-C. Width, length and doubling times obtained from OD600 measurements of MG1655, TKL130, and TKL130/pKC128 cells.
Figure 1—figure supplement 1D Bocillin labeling, fluorescence and mass spectrometry measurements of MG1655, TKL130, and TKL130/pKC128 cells.
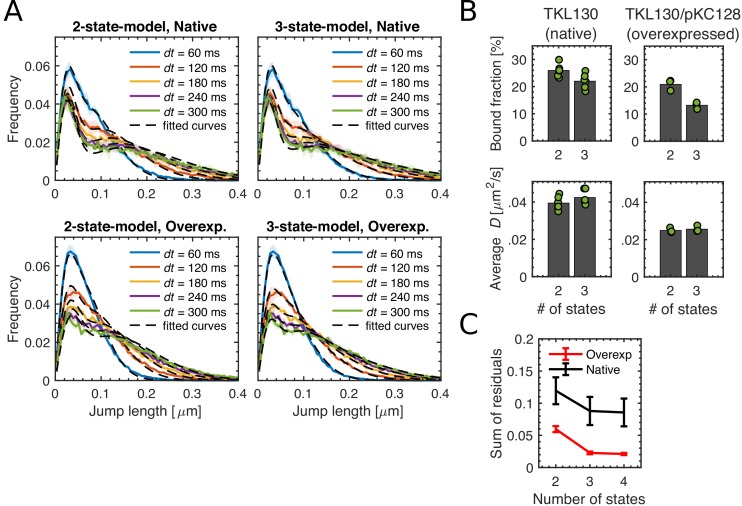
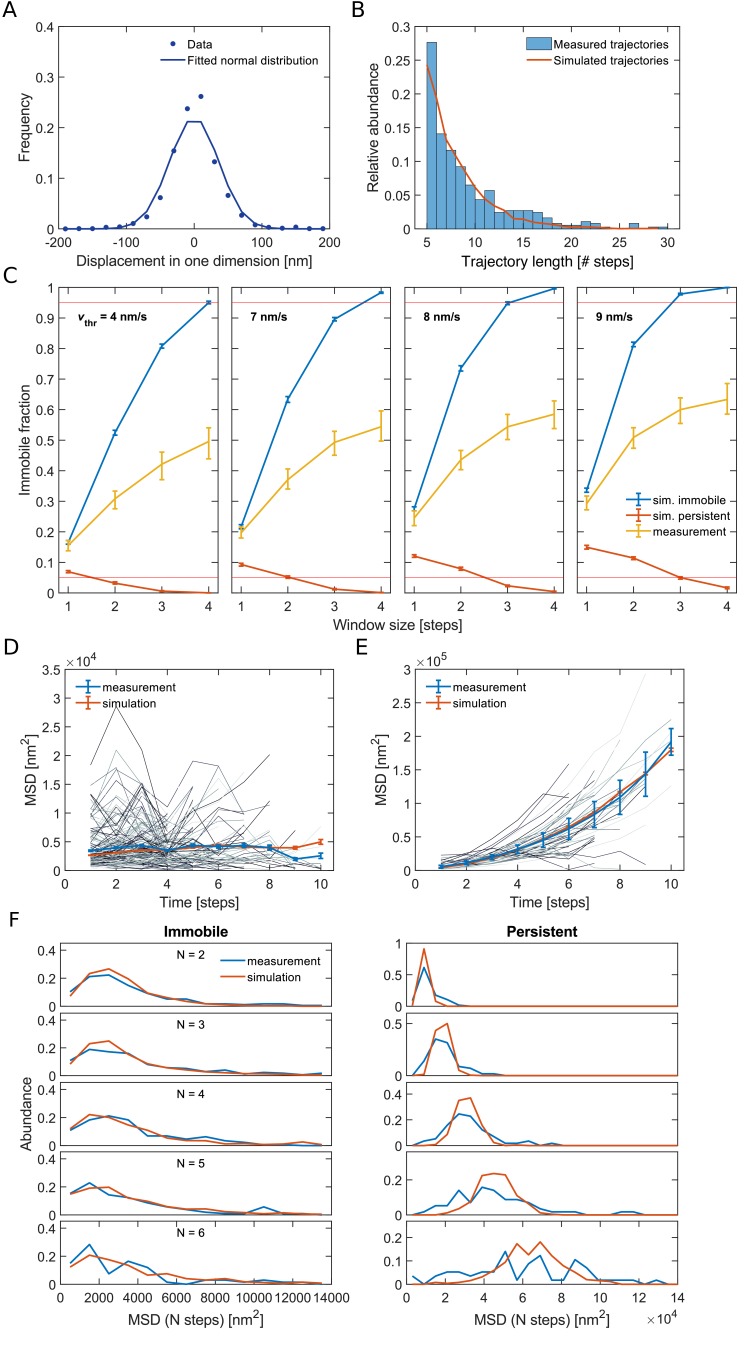
Figure 1—figure supplement 2 Values of bound fractions, average diffusion constants, and sum of residuals of PAmCherry-PBP2 tracks from TKL130/pKC128 strain with high frequency imaging given by the Spot-On method using 2- or 3-state diffusion model.
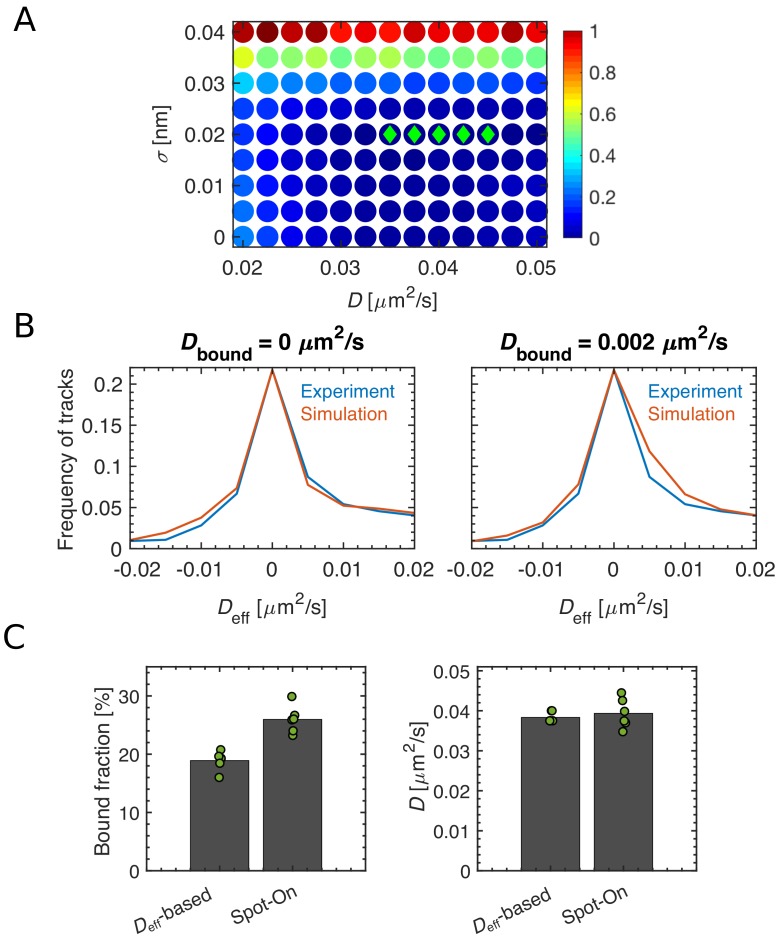
Figure 1—figure supplement 3 Values of bound fractions and diffusion constants of PAmCherry-PBP2 tracks from TKL130/pKC128 strain to compare the results from Spot-On with the
Deff based method.
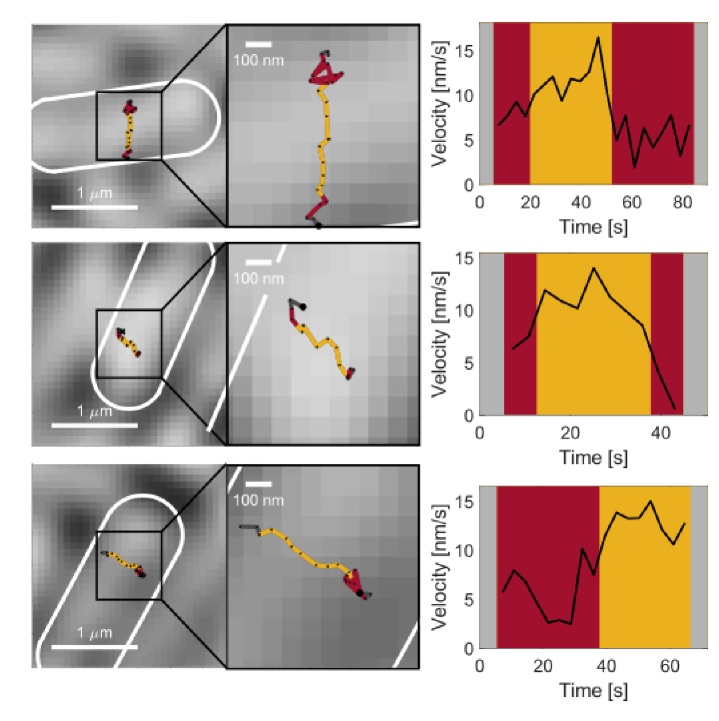
Figure 1—figure supplement 5 Raw data for
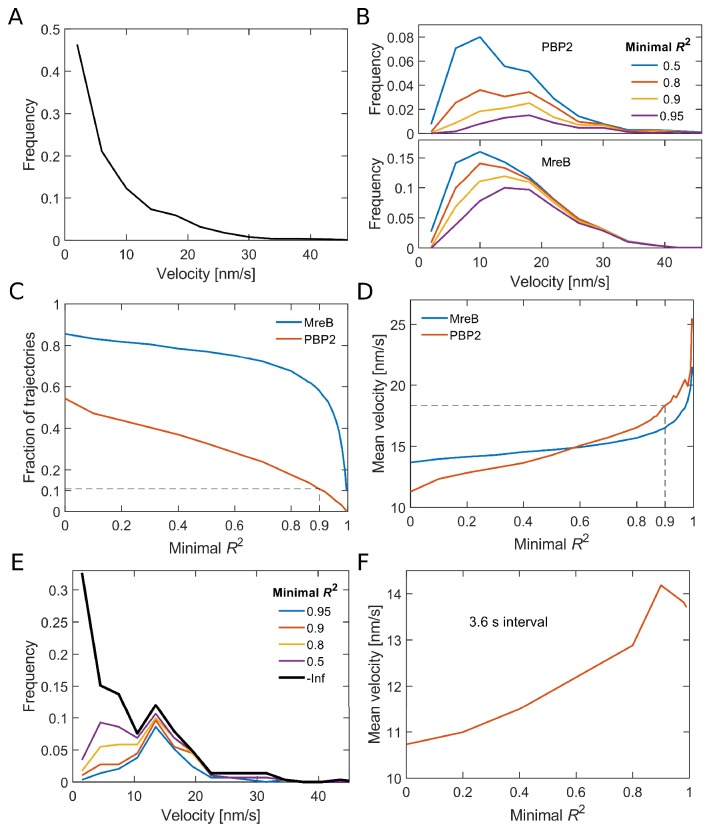
Figure 1F–G. List of velocity, orientation and goodness of fit (R
2).
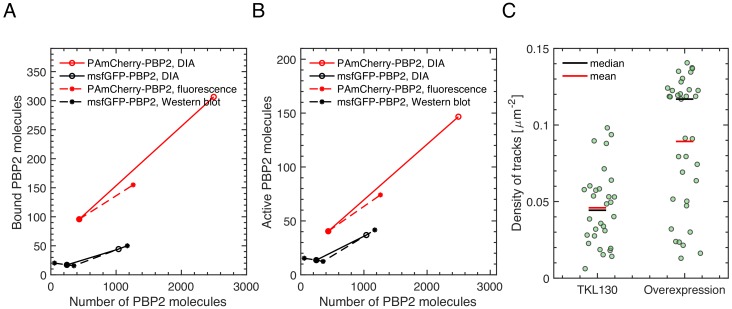
Figure 1—figure supplement 7 Number of bound or active PBP2 molecules as a function of the number of PBP2 molecules per cell acquired from mass spectrometry and fluorescence measurements.
Figure 1—figure supplement 8A) Shape and OD600 measurements of cells carrying the msfGFP-PBP2 fusion expressed from the native or P
Lac promoters with different induction levels of IPTG.
Figure 1—figure supplement 8B Fluorescence and Western Blot quantifications and mass spectrometry (both DIA and PRM) of cells carrying the msfGFP-PBP2 fusion expressed from the native or P
Lac promoters with different induction levels of IPTG
Figure 1—figure supplement 8C Values of number of tracks, average diffusion constants, and bound fractions for cells carrying the msfGFP-PBP2 fusion, including p-values for bound fractions.
Figure 1—figure supplement 9 Shape and fluorescence measurements, number of tracks, average diffusion constants and bound fractions from high frequency imaging of cells carrying msfGFP-PBP2 fusion with induction levels of 5 and 25 uM IPTG after different times of growth, including p-values for bound fractions.
Figure 1—figure supplement 10A Values of number of tracks, and persistent fractions for cells carrying msfGFP-PBP2 fusion expressed from the native or P
Lac promoters with different induction levels of IPTG, including p-values for persistent fractions.
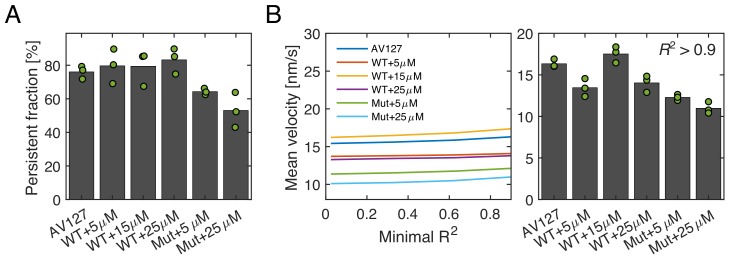
Figure 1—figure supplement 10B. List of velocity and goodness of fit (R
2) values for cells carrying msfGFP-PBP2 fusion under different induction levels of IPTG.