Abstract
Background
The human gut microbiome is recognized as an important determinant of human health, yet little is known about how dietary habits are related to the microbiome in post-weaned, pre-pubescent children.
Objective
The goal of this work was to link quantitative dietary intake with microbiome features in a diverse population of children consuming a predominantly Western diet.
Design
This was a cross-sectional study.
Participants/settings
English or Spanish-speaking families with healthy children between the ages of 2–9 years were recruited from a community-based, early childhood learning center in suburban Los Angeles, California between June and September 2014.
Main Outcome Measures
Children included in the analyses (n=75) contributed three fecal samples and three, quantitative 24-hour dietary recalls using the multiple pass method with an average of 5.7 days between samples. Microbial communities of each fecal sample were characterized using Illumina sequencing of the 16S rRNA gene. Dietary recalls were analyzed using the Automated Self-administered 24-hour recall (ASA24) Dietary Assessment Tool.
Statistical Analysis Performed
Associations between dietary factors and microbiome features were assessed using the Kruskal-Wallis test, Spearman rank correlations, or PERMANOVA. For demographic and health-related variables, Chi-square analyses were used to test for differences between age groups for categorical variables.
Results
Results show that age is correlated with three metrics of microbiome diversity (P < 0.05), and is associated with both community structure (P = 0.0488) and membership (P = 0.0002). Several dietary food groups and nutrients were likewise associated with microbiome features. For example, consumption of non-whole grain foods was associated with community structure (P = 0.0089) and membership (P = 0.0057), but not diversity (P > 0.05). Likewise, the relative abundance of several bacterial taxa were linked to consumption of particular food groups and/or nutrients as illustrated by the positive associations between total fruit (PFDR < 0.05) and fiber (PFDR < 0.05) consumption with the relative abundance of the Lachnospira genera.
Conclusion
This hypothesis-generating study demonstrates that the composition of the child gut microbiome remains dynamic beyond the age of 3 years and responds to dietary differences across individuals. In particular, non-whole grain foods fortified with vitamins and minerals appear to be associated with the composition of the microbiome. Future interventional or model organism-based studies will be needed to test these associations between diet and microbiome composition.
Keywords: gut microbiome, 16S rRNA gene sequencing, childhood nutrition, fortified grains, dietary composition
INTRODUCTION
The human gut harbors trillions of microbial cells that encode a gene set 150 times larger than their human hosts1. Collectively known as the human gut microbiota, these microorganisms play a key role in promoting and maintaining human health by performing essential functions throughout ones life. For example, the gut microbiota helps develop the immune system, aids in energy harvest from food, synthesizes vitamins, and protects from invading pathogens2,3. Despite these essential functions, the taxonomic composition4 and stability5 of each individual’s microbiome is distinct. The individualized nature is related to a number of factors6, but diet in particular has been suggested as one of the most important determinants of gut microbiome composition7.
The human gut microbiome is largely founded at birth8 with delivery mode determining which microbes colonize first9. After this initial colonization, development of the gut microbiome continues into infanthood and is heavily affected by diet. For example, breastfed infants host different gut microbiomes than formula fed infants10. Once solid foods are introduced, the infant microbiome undergoes an abrupt shift in community composition which ultimately leads to an adult-like community by about the age of 311–16. Beyond this life stage and prior to adulthood, comparably little is known how diet is related to the microbiome. Recently, Berding and colleagues were able to link broad-scale dietary patterns to gut microbiome composition and stability in a population of 4- to 8-year old American children17. They found that children could be separated into two broad scale dietary groups largely determined by consumption of fish, refined carbohydrates, vegetables, and convenience foods like juice and snacks. These dietary groups were associated with differences in microbiome composition highlighted by differential abundance of key bacterial genera like the Bacteroides, Prevotella, and Bifidobacterium. In adulthood, both short-18 and long-term19,20 dietary habitats are known to influence diversity and composition of the gut microbiome. Clearly, dietary habits are an important factor structuring the gut microbiome throughout life.
Over the last 50 years’ global dietary patterns have shifted due to a rapid transition to a ‘Western diet’. A Western diet can broadly be defined by an increased consumption of refined re-fortified carbohydrates, high-fat animal-products, and highly processed foods21. This shift in diet has been connected to the current and expanding obesity epidemic with over 65% of American adults being overweight or obese22, resulting in an increase in heart disease23, type 2 diabetes24, and multiple gastrointestinal diseases25. Dysbiosis of the human gut microbiome has been connected to all of the aforementioned diseases26–28, suggesting that this dietary change has a detrimental effect on the microbiome-host relationship. Although it is unclear if the dysbiosis is the cause or consequence of the disease state, dysbiosis effectively changes the composition of the gut microbiota having potentially detrimental effects on human physiology29. The gut microbiome has also been suggested to play a role in these inflammatory diseases through the production of pro-inflammatory compounds that leak from the gut causing chronic low-grade inflammation30,31. Consumption of a ‘Western diet’ has been shown to result in a less diverse gut microbiome, when compared to a more traditional rural diet32,33.
Seeing that diet is an important factor modulating the composition of the gut microbiota in infants and adults, a comprehensive understanding of these interactions across all life-stages in healthy populations is needed. The purpose of this study was to examine the relationship between diet and gut microbiome composition of post-weaned, pre-pubescent American children aged 2–9 years. The primary objective was to link quantitative dietary intake data with microbiome diversity, composition, and taxonomy to generate hypotheses for future intervention or model organism-based studies.
METHODS
Ethics
The Institutional Review Board at California State University, Northridge approved the study protocol (#1314–223). All adult participants provided written informed consent for their children to participate in the study and children provided verbal assent.
Data availability
Raw sequence data, accompanying metadata, and all supplemental data are available online at figshare34. Sequence data are also available through Qiita35,36 (QIITA: 12360).
Recruitment
Participants were recruited in the summer of 2014 at an early childhood education center in Canoga Park, California, a suburb north of Los Angeles. Eligibility criteria for the study were; English or Spanish-speaking parents, families with a child between the ages of 2–9 years, weaned from breastfeeding, and no known communicable diseases at the time of recruitment. Families were also excluded if in the three months prior to the screening interview, children had taken antibiotics, had excessive vomiting or diarrhea, or blood or mucus in their stool. A brief survey collecting demographic and health information (age, height, weight, ethnicity, etc.) was administered after signed consent (https://doi.org/10.6084/m9.figshare.7011272.v1). Participants were asked to provide three fecal samples and accompanying dietary information (see below) within a two-month time window. Upon completion of data collection, volunteers were given a $20 gift card as compensation for their participation.
Microbiome sampling, DNA extraction, PCR and Sequencing
Fecal samples were collected by parents in the privacy of their homes using sterile, double-tipped swabs by swabbing toilet paper or diapers after use. This protocol is minimally invasive and has been successfully used in other community-based research projects4,5,37. Although feces may not provide the best representation of the spatial heterogeneity of the gut microbiome, it is the best and only option we have to study these communities in healthy individuals. Nucleic acids (DNA) were extracted directly from swabs using the MoBio PowerSoil DNA isolation kit following the manufacturer’s protocol with slight modifications that allow for robust yields38. Polymerase chain reaction (PCR) amplification of the variable region 4 (V4) of bacterial and archaeal 16S rRNA genes was performed using the barcoded primer set (515F/806R), PCR mixture conditions, and thermal cycling steps previously described39. PCR amplicons of triplicate reactions for each sample were quantified and pooled at approximately equal amounts, cleaned using a single-tube MoBio Ultraclean PCR Clean-up Kit (MoBio Laboratories, Carlsbad, CA USA), and sequenced using an Illumina MiSeq (v2, 2 × 150bp) instrument.
Sequence data processing
Raw fastq files of 16S rRNA genes were processed using the UPARSE pipeline40 with a few modifications. Briefly, a custom Python script was used to demultiplex and prepare sequence files for paired-end assembly and clustering. Paired sequences were assembled using UPARSE with the following parameters: fastq_truncqual 3, fastq_maxdiffs 1, fastq_minovlen 20, fastq_minmergegelen 200. Assembled sequences were filtered at a maxee value of 0.5, which means that, on average, only one nucleotide in every two sequences is potentially incorrect. Quality sequences were then dereplicated and singletons were removed. Operational taxonomic units (OTUs) were assigned with UPARSE at a 97% sequence identity threshold. Taxonomy was assigned to representative sequences of each OTU using the RDP classifier41 with a confidence threshold of 0.5 against the Greengenes 13_8 database42,43 as implemented in QIIME v. 1.9.044. QIIME was also used to generate phylogenetic trees using FastTree45 and to calculate various alpha- (richness, phylogenetic diversity46, and the Shannon Diversity Index) and beta-diversity (UniFrac47) metrics. The two beta diversity metrics used, weighted and unweighted UniFrac, provide different insights into microbiome community composition. The unweighted UniFrac metric accounts only for the presence and absence of OTUs in each pair of samples and is thus considered to measure community membership. In contrast, the weighted UniFrac metric also accounts for the relative abundance of each OTU and is thus considered to measure community structure.
After quality filtering, a total of 3,810,822 sequences were generated across 349 samples. After rarefaction (n=3,772 sequences/sample), alpha diversity metrics were calculated and averaged across the three samples per individual. To determine the average composition (beta diversity) and taxonomy across the three time points, we used the collapse_samples.py command in QIIME with the --collapse_mode option set to ‘mean’ after rarefaction44. Beta diversity metrics and taxonomic summaries were generated using the collapsed OTU table.
Dietary assessment
Children’s dietary information and supplement intake was collected using a paper instrument with parents as proxies for children’s intakes. Parents were considered the best proxies for these young children, as they were with them during the day and were the ones to feed them and observe their eating habits. To assist with recall, parents were first asked to record a food list at home for the 24-hour recall period that was assessed in person at the early childhood learning center. The food list was used as a memory prompt for parents when collecting the actual 24-hour recall in person, which was interviewer-administered. A total of three, non-consecutive, quantitative, 24-hour recalls using the multiple pass method were collected from each participant, one time weekly and included both weekdays and one weekend day. Twenty-four hour dietary recalls were chosen to assess recent dietary intake because they have been shown to be a valid measure for this age group when compared to measures using doubly-labeled water48–50. At the end of the 24-hour recall, a question was posed asking if the child was taking any supplements and to indicate which one(s). The 24-hour recalls and supplement data were manually entered in the Automated Self-administered 24-hour recall (ASA24) Dietary Assessment Tool, version 2016 (https://epi.grants.cancer.gov/asa24/), an electronic data collection and dietary analysis program The ASA24 employs research-based strategies to enhance dietary recall using: 1) a respondent-driven approach allowing initial recall to be self-defined; 2) association with the day’s events; 3) probes for frequently forgotten foods; 4) repetition with minimal burden; 5) reviews 24-hour day; and 6) placement of foods with eating occasions. These steps were emulated in the interviewer-administered version of the 24-hour dietary recall with parents and the question regarding supplement use was reviewed at that time with the parent respondent. Individual-level nutrient and food group estimates from the ASA2451 were compared to dietary reference intakes (DRIs) as well as food group recommendations based on the Dietary Guidelines for Americans52. Macronutrients were compared to the acceptable macronutrient distribution ranges (AMDRs) by age and macro-(where appropriate) and micronutrients were compared to the estimated average requirements (EARs) by age. Food group data were provided by the MyPyramid Equivalents database (MPED v2)53 and the Center for Nutrition Policy and Promotion’s fruit database (03–04 version)54 and were calculated by the ASA24 program as described in the ASA24 researcher instructions guide51. Food groups calculated included grains in ounce equivalents (total, whole, and non-whole/refined), vegetables in cup equivalents (total of all groups, dark green, orange, white potatoes, other starchy vegetables, tomatoes and other starchy vegetables), fruits in cup equivalents (total of all groups, citrus fruits melons and berries, other, and whole), milk in cup equivalents (total of all groups, milk, yogurt, cheese), meat and beans in ounce equivalents (meat, poultry and fish together, meat, organ meats, frankfurters, sausage and luncheon meats, poultry, fish and shellfish high in n-3 fatty acids, fish and shellfish low in n-3 fatty acids, eggs, cooked dried peas and beans, soybean products (tofu and meat analogs), and nuts and seeds. Disaggregated dietary data averaged across the three time points for healthy children (n=75) are available at figshare34.
Statistical analysis
The non-parametric Kruskal Wallis test was used to determine if the sex of a child was related to overall microbiome diversity. Spearman rank correlations were used to investigate relationships between microbiome diversity and taxonomy with age and dietary features using the ‘corr.test’ function in the R55 package psych56. Correlation heatmaps were plotted using the corrplot 57 package also in R. To determine if dietary features are associated with community membership (unweighted UniFrac) or structure (weighted UniFrac), the ‘adonis’ function (i.e. PERMANOVA) with 9,999 permutations from the R vegan58 package was used. Also in vegan, the ‘envfit’ function was run with 9,999 permutations to find correlations between dietary features and gut microbiome composition in ordination space. Dietary variables with P-values less than 0.05 were overlaid as directional vectors on non-metric multidimensional scaling (NMDS) plots to visualize correlations. Because of the exploratory nature of the study and nested structure of many dietary components (e.g. whole grains are part of total grains), uncorrected and corrected (Benjamini and Hochberg FDR correction59) P-values are presented when appropriate. For demographic and dietary variables, Chi-square analyses were used to test for differences between age groups for categorical variables. Percentages of recommended intakes by age are provided for the Dietary Reference Intakes (DRIs)60. Acceptable Macronutrient Distribution Ranges (AMDRs)61, Estimated Average Requirements (EARs)60, and Adequate Intakes (AIs)60 and supplement use.
Sample selection
In total, 130 individuals provided consent and at least one fecal sample and 24-hour dietary recall. Of these, 94 provided all three fecal samples and 24-hour dietary recalls. Since the focus of this study was to examine how dietary factors are associated with the gut microbiome in healthy children, the study population was filtered further to exclude subjects that self-reported conditions previously found to be associated with microbiome dysbiosis. Exclusion criteria included asthma, eczema, colitis, autism spectrum disorder including pervasive developmental disorder not otherwise specified (PDD-NOS), and epilepsy. The remaining 75 children were included in the analyses presented here. The research protocol was to obtain microbiome samples and dietary data, once weekly. However, the number of days between data collection varied due to participant convenience. As a result, the number of days between sample collections ranged from 1 to 42 with an average of 5.7 (median = 4) days.
RESULTS
Demographics and dietary intake
Table 1 shows the demographic characteristics of the analytical sample by age group. The majority of study participants were female (60%), of Hispanic ethnicity (50.7%), had family incomes less than $20,000/year (30.7%), participated in the Women, Infants, and Children (WIC) program (88.9%), had a normal weight according to their body mass index (BMI) percentile (62.7%), and had been breastfed (84%). The only difference between the age groups (2–3 vs. 4–9) in terms of their sociodemographic factors was that more children 4–9 years were in the lowest income category compared to those who were 2–3 years of age.
Table 1.
Demographics, program participation and health information of 75 children recruited from a community-based, early childhood learning center in suburban Los Angeles, California by age group. Results of Pearson’s chi-squared test comparing various categorical variables across age groups are also presented.
| Age | ||||
|---|---|---|---|---|
| 2 – 3 years n (%) (n = 30) |
4 – 9 years n (%) (n = 45) |
Chi-square | P value | |
| Sex, n (%) | ||||
| Female | 17 (56.7) | 28 (62.2) | 0.23 | 0.63 |
| Male | 13 (43.3) | 17 (37.8) | ||
| Race/Ethnicity, n (%) | ||||
| White | 10 (33.3) | 5 (11.1) | ||
| African-American | 1 (3.3) | 1 (2.2) | ||
| Hispanic | 12 (40) | 26 (57.8) | 5.93 | 0.20 |
| Asian or Pacific Islander | 2 (6.7) | 3 (6.7) | ||
| Othera | 5 (16.7) | 10 (22.2) | ||
| Household income, n (%) | ||||
| < $20,000 | 4 (16) | 19 (45.2) | ||
| $20,000 – $29,000 | 3 (12) | 9 (21.4) | 11.72 | 0.008 |
| $30,000 - $59,000 | 7 (28) | 9 (21.4) | ||
| ≥ $60,000 | 11 (44) | 5 (11.9) | ||
| WIC,b n (%) | ||||
| Yes | 12 (40) | 28 (62.2) | 3.57 | 0.06 |
| No | 18 (60) | 17 (37.8) | ||
| SNAP,c n (%) | ||||
| Yes | 4 (13.3) | 8 (17.8) | 0.26 | 0.61 |
| No | 26 (86.7) | 37 (82.2) | ||
| BMI,d Percentile, n (%)d | ||||
| Underweight | 1 (3.3) | 4 (8.9) | ||
| Normal weight | 20 (66.7) | 27 (60) | 3.06 | 0.383 |
| Overweight | 3 (10) | 9 (20) | ||
| Obese | 6 (20) | 5, 11.1 | ||
| Ever Breastfed, n (%) | ||||
| Yes | 25 (83.3) | 38 (84.4) | 0.17 | 0.898 |
| No | 5 (16.7) | 7 (15.6) | ||
Other race/ethnicity includes: Armenian, East Indian, Spanish and mixed race
WIC: Special Supplemental Nutrition Program for Women, Infants and Children
SNAP: Supplemental Nutrition Assistance Program; BMI: Body Mass Index calculated as kg/m2
BMI percentiles were calculated using the Centers for Disease Prevention and Control (CDC) growth chart data and calculation tools. Available at: https://www.cdc.gov/healthyweight/assessing/bmi/childrens_bmi/tool_for_schools.html
Table 2 shows the comparison of average dietary intake including intake of dietary supplements by age group and the dietary reference intakes, specifically the percent above and below the estimated average requirements (EARs)60. All dietary data were assessed with respect to meeting participants’ age-specific requirements. Study participants reported the lowest intakes compared to EARs/AIs for fiber, calcium, potassium, choline, and the fat-soluble vitamins, D, E, and K, with smaller differences for magnesium, phosphorus, vitamins A and B1260. Participants also reported dietary intakes in excess of the EARs/AIs for all but two of the nutrients analyzed60. Excesses greater than two times the EARs were reported for more than 90% of study participants for: protein, iron, selenium, riboflavin, pyridoxine, and vitamin B12. Macronutrient intakes were also compared to DRIs using the acceptable macronutrient distribution ranges (AMDRs)61 by age group (data not shown). AMDRs were exceeded by 2.6% of study participants for protein, 6.7% for carbohydrates, 5.3% for total fat, and 82.7% for saturated fat. Intakes lower than AMDRs were reported for 28% of study participants for total fat.
Table 2.
Comparison of average dietary intake of 75 children recruited from a community-based, early childhood learning center in suburban Los Angeles, California by age group across three, 24-hour dietary recalls using USDA’s multiple pass method –dietary reference intakes: percent above and below the estimated average requirements by age.
| Age Groups | Estimated Average Requirements (EAR) | |||||
|---|---|---|---|---|---|---|
| 2 – 3 y Mean ± SD (min – max) |
4 – 8 y Mean ± SD (min – max) |
2 – 3 y | 4 – 8 y | Percent (%) above EARa | Percent (%) below EARb | |
| Energy (kcal) | 1369.6 ± 314.2 (890.6 – 2225.5) |
1467.6 ± 334.9 (782.8 – 2145.5) |
||||
| Protein (g/kg/d) | 3.43 ± 1.01 (2.07 – 5.42) |
2.90 ± 1.05 (1.26 – 7.28) |
0.87 | 0.76 | 96 | - |
| Fat (g/d) c | 47.3 ± 14.9 (28.0 – 83.9) |
49.2 ± 13.3 (19.4 – 80.3) |
ND | ND | - | |
| Carbohydrates (g/d) | 191.7 ± 52.6 (131.2 – 361.0) |
203.1 ± 51.8 (117.3 – 332.9) |
100 | 100 | 42.7 | - |
| Fiber (g/d) d | 13.9 ± 4.5 (5.8 – 26.0) |
14.3 ± 6.0 (5.9 – 34.0) |
19 | 25 | - | 90.7 |
| Calcium (mg/d) | 910.8 ± 218.7 (533.4 – 1525.1) |
845.6 ± 271.1 (399.2 – 1519) |
500 | 800 | 10.7 | 29.3 |
| Copper (μg/d) | 950 ± 260 (610 – 1,690) |
970±310 (440 – 1,770) |
260 | 340 | 85.3 | - |
| Iron (mg/d) | 11.1 ± 4.16 (5.53 – 19.9) |
13.5 ± 4.47 (7.10 – 24.7) |
3 | 4.1 | 90.7 | - |
| Magnesium (mg/d) | 216.0 ± 44.5 (138.8 – 310.4) |
218.2 ± 57.3 (122.2 – 337.6) |
65 | 110 | 64 | 1.3 |
| Phosphorus (mg/d) | 1006.3± 203.0 (561.6 – 1465.6) |
1037.9 ± 243.2 (583.1 – 1538.5) |
380 | 405 | 82.7 | 2.7 |
| Potassium (g/d) d | 2.07 ± 0.48 (1.30 – 3.62) |
2.11 ±0.64 (1.19 – 3.52) |
2.0 | 2.3 | - | 54.7 |
| Selenium (μg/d) | 64.7 ± 16.5 (34.4 – 95.0) |
78.5 ± 22.4 (40.3 – 160.8) |
17 | 23 | 94.7 | - |
| Sodium (mg/d) d | 1898.8 ± 461.6 (1322.7 – 2961) |
2275.1 ± 581.6 (1159 – 3758) |
800 | 1,000 | 69.3 | - |
| Zinc (mg/d) | 8.32 ± 2.22 (5.36 – 14.6) |
9.45 ±3.19 (4.53 – 20.1) |
2.5 | 4 | 77.3 | - |
| Vitamin A (RAE) e (μg/d) | 450.2 ± 156.3 (194.8 – 761.4) |
438.6 ± 175.7 (107.6 – 1115.9) |
210 | 275 | 68 | 4.0 |
| Thiamin (B1) (mg/d) | 1.23 ± 0.42 (0.58 – 2.58) |
1.36 ± 0.38 (0.69 – 2.33) |
0.4 | 0.5 | 86.7 | - |
| Riboflavin (B2) (mg/d) | 1.82 ± .43 (1.12 – 2.95) |
1.85 ± .53 (0.92 – 3.20) |
0.4 | 0.5 | 97.3 | |
| Niacin (B3) (mg/d) | 14.7 ± 5.17 (5.36 – 28.6) |
17.3 ± 5.31 (8.66 – 31.00) |
5 | 6 | 77.3 | - |
| Pyridoxine (B6) (mg/d) | 1.52 ± 0.58 (0.57 – 2.96) |
1.62 ± 0.57 (0.93 – 3.02) |
0.4 | 0.5 | 92.0 | - |
| Folate (B9) (μg/d) | 314.8 ± 110.3 (123.5 – 616.4) |
362.3 ± 137.9 (182.2 – 715.0) |
120 | 160 | 58.7 | - |
| Vitamin B12 (μg/d) | 4.31 ± 1.78 (1.22 – 8.67) |
5.66 ± 7.36 (0.93 – 52.36) |
0.7 | 1.0 | 96 | 1.3 |
| Vitamin C (mg/d) | 79.6 ± 36.8 (15.3 – 173.1) |
92.4 ± 60.2 (23.9 – 361.0) |
13 | 22 | 82.7 | - |
| Vitamin D (μg/d) | 5.77 ± 2.55 (1.57 – 12.9) |
5.05 ± 2.36 (0.51 – 11.3) |
10 | 10 | - | 96.0 |
| Vitamin E (mg/d) | 6.32 ± 3.61 (2.02 – 15.4) |
5.28 ± 2.55 (1.33 – 12.7) |
5 | 6 | 6.5 | 65.3 |
| Vitamin K (μg/d) d | 73.1 ± 66.9 (18.3 – 307.1) |
59.5 ± 55.5 (9.98 – 294.2) |
30 | 55 | 20 | 52.0 |
| Choline (mg/d) d | 210.7 ± 60.8 (81.2 – 329.9) |
222.8 ± 74.1 (106.5 – 472.4) |
200 | 250 | - | 60.0 |
Percents refer to proportions of participants with dietary intakes greater than or equal to 2 times (200%) the EAR. Two times the EAR (200%) was chosen as the cut-off because more than 54% of the nutrients analyzed in the table had EARs above 100%.
Percents refer to proportions of participants with dietary intakes below 100% of EAR.
Total fat and saturated fat are compared to the Acceptable Macronutrient Distribution Ranges (AMDRs), which are 30%–40% for children 1 – 3 years of age, 25%–35% for children ages 4 – 18 years (total fat) and < 10% for children of all ages (saturated fat).
Adequate Intakes (AI) used for nutrients without EARs. For sodium, 30.7% of participants reported intakes greater than 100% of the AI, but less than 200%.
RAE: retinol activity equivalents.
A total of 37.3% of participants (n=28) reported taking some type of dietary supplement. These included multivitamin/multimineral supplements (60.7%), calcium (3.6%), omega-3s (3.6%), zinc (3.6%), minerals alone (3.6%), and Pediasure (3.6%). Two participants reported taking supplements but did not specify which type (7.1%). One participant reported taking a probiotic supplement. Data for the relationship with the microbiome were analyzed with and without this individual. Because no differences were identified with this participant included, they were not removed from these analyses.
Microbiome
Alpha diversity
With all three metrics tested (Shannon Index, PD, and richness), diversity was positively associated with age meaning that older children tended to have more diverse microbiomes than younger children (Table 3). In contrast, sex was not significantly associated with diversity in this population of children. To investigate which dietary components were associated with diversity of the child gut microbiome, dietary data was disaggregated and split into food groups (fruits, vegetables, grains, dairy, and proteins) and micro- and macronutrients. Protein consumption, split into plant and animal-based proteins, were the only food groups correlated with any of the alpha-diversity (Shannon index, PD, and richness) metrics (Table 3). When food groups were further split into subcategories, consumption of meat, poultry, and yogurt showed significant relationships with two of the three alpha diversity metrics. With the exception of selenium (P=0.014) and niacin (P=0.037), which were correlated with the Shannon index, macronutrients and micronutrients were not related to microbiome alpha-diversity (Table 4).
Table 3.
Results of PERMANOVA and Spearman rank correlations between selected demographic features and dietary food groups with gut microbiome community structure (weighted UniFrac), community membership (unweighted UniFrac), and three measures of diversity in a population of children aged 2–9 years (n=75) recruited from a community-based, early childhood learning center in suburban Los Angeles, California. Explained variance for PERMANOVA analysis is expressed as R2 values for 9,999 permutations. P values in italics remain significant after correction for multiple comparisons (corrected P ≤ 0.05).
| Weighted UniFrac | Unweighted UniFrac | Shannon Index | Phylogenetic Diversity | Richness | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Factor | R2 | P value | R2 | P value | Rho | P value | Rho | P value | Rho | P value |
| Age | 0.027 | 0.0488 | 0.035 | 0.0002 | 0.379 | <0.001 | 0.242 | 0.037 | 0.340 | 0.003 |
| Sex | 0.006 | 0.9329 | 0.014 | 0.3221 | 0.073a | 0.787 | 0.003a | 0.957 | 0.054a | 0.816 |
| Fruit | 0.033 | 0.0169 | 0.015 | 0.2242 | 0.021 | 0.856 | −0.110 | 0.349 | −0.057 | 0.628 |
| Citrus/melon/berriesb | 0.043 | 0.0071 | 0.015 | 0.2365 | −0.025 | 0.831 | −0.131 | 0.262 | −0.011 | 0.925 |
| Other fruit | 0.008 | 0.8034 | 0.017 | 0.1097 | 0.027 | 0.818 | −0.130 | 0.268 | −0.137 | 0.242 |
| Grain | 0.038 | 0.0059 | 0.023 | 0.0176 | 0.098 | 0.400 | −0.070 | 0.550 | 0.011 | 0.924 |
| Whole grain | 0.021 | 0.1134 | 0.017 | 0.112 | −0.088 | 0.451 | −0.085 | 0.470 | −0.117 | 0.317 |
| Non-whole grain | 0.037 | 0.0089 | 0.025 | 0.0057 | 0.141 | 0.226 | −0.023 | 0.843 | 0.070 | 0.551 |
| Vegetable | 0.024 | 0.0746 | 0.021 | 0.0261 | 0.145 | 0.216 | −0.015 | 0.901 | 0.027 | 0.816 |
| Dark green | 0.010 | 0.5985 | 0.013 | 0.5146 | 0.047 | 0.692 | −0.052 | 0.657 | 0.019 | 0.872 |
| Orange | 0.008 | 0.7730 | 0.014 | 0.3535 | −0.072 | 0.538 | −0.136 | 0.245 | −0.177 | 0.129 |
| Potato (white) | 0.024 | 0.0771 | 0.023 | 0.0144 | 0.002 | 0.983 | 0.048 | 0.684 | 0.045 | 0.699 |
| Starchyc | 0.020 | 0.1439 | 0.013 | 0.5212 | 0.024 | 0.841 | −0.037 | 0.752 | −0.033 | 0.779 |
| Tomato | 0.042 | 0.0057 | 0.021 | 0.0329 | 0.034 | 0.772 | 0.001 | 0.993 | 0.014 | 0.903 |
| Other | 0.010 | 0.6216 | 0.018 | 0.1108 | 0.080 | 0.493 | −0.119 | 0.308 | −0.060 | 0.609 |
| Dairy | 0.009 | 0.6928 | 0.011 | 0.7236 | −0.036 | 0.758 | −0.053 | 0.652 | −0.129 | 0.270 |
| Milk | 0.019 | 0.1710 | 0.015 | 0.2694 | −0.036 | 0.761 | 0.069 | 0.558 | −0.033 | 0.782 |
| Yogurt | 0.014 | 0.3852 | 0.023 | 0.0147 | −0.158 | 0.176 | −0.311 | 0.007 | −0.218 | 0.060 |
| Cheese | 0.013 | 0.4364 | 0.016 | 0.1923 | 0.035 | 0.769 | −0.190 | 0.102 | −0.157 | 0.179 |
| Protein – animald | 0.020 | 0.1506 | 0.019 | 0.0593 | 0.282 | 0.014 | 0.151 | 0.196 | 0.224 | 0.054 |
| Meate | 0.020 | 0.1533 | 0.019 | 0.0809 | 0.301 | 0.009 | 0.142 | 0.224 | 0.225 | 0.052 |
| Meat (no poultry or fish) | 0.021 | 0.1233 | 0.012 | 0.5502 | 0.099 | 0.399 | 0.047 | 0.688 | 0.108 | 0.355 |
| Organ meat | 0.009 | 0.6454 | 0.012 | 0.5829 | 0.000 | 1.000 | −0.081 | 0.492 | −0.086 | 0.464 |
| Processed meatsf | 0.017 | 0.2242 | 0.013 | 0.5007 | 0.025 | 0.829 | 0.010 | 0.931 | 0.060 | 0.606 |
| Poultry | 0.012 | 0.5182 | 0.017 | 0.1186 | 0.350 | 0.002 | 0.122 | 0.299 | 0.191 | 0.102 |
| Seafood high fatty acids | 0.008 | 0.7110 | 0.013 | 0.4767 | −0.026 | 0.822 | −0.097 | 0.408 | −0.118 | 0.315 |
| Seafood low fatty acids | 0.019 | 0.1775 | 0.009 | 0.9808 | −0.055 | 0.640 | −0.014 | 0.906 | −0.048 | 0.685 |
| Eggs | 0.009 | 0.7480 | 0.014 | 0.3919 | 0.060 | 0.609 | 0.103 | 0.378 | 0.139 | 0.235 |
| Protein – plantg | 0.030 | 0.0301 | 0.019 | 0.0534 | −0.308 | 0.007 | −0.055 | 0.637 | −0.137 | 0.239 |
| Soy | 0.014 | 0.3658 | 0.025 | 0.0185 | −0.032 | 0.788 | −0.059 | 0.617 | −0.039 | 0.740 |
| Nuts/seeds | 0.023 | 0.0961 | 0.027 | 0.0051 | −0.214 | 0.065 | −0.204 | 0.078 | −0.226 | 0.051 |
| Legumes | 0.025 | 0.063 | 0.017 | 0.1139 | −0.057 | 0.630 | 0.200 | 0.086 | 0.102 | 0.383 |
chi-squared values from Kruskal-Wallis test
includes juices
other than potatoes, peas and beans (legumes)
sum of M_MPF and eggs
includes beef, pork, veal, lamb, game, organ meats, sausages, luncheon meat, poultry, and fish and shellfish
frankfurters, sausages, luncheon meat.
sum of nuts, soy, and legumes (converted to oz).
Table 4.
Results of PERMANOVA and Spearman rank correlations between dietary nutrient intake and gut microbiome community structure (weighted UniFrac), community membership (unweighted UniFrac), and three measures of diversity in a population of children aged 2–9 years (n=75) recruited from a community-based, early childhood learning center in suburban Los Angeles, California. Explained variance for PERMANOVA analysis is expressed as R2 values for 9,999 permutations. P values in italics remain significant after correction for multiple comparisons (corrected P ≤ 0.05).
| Weighted UniFrac | Unweighted UniFrac | Shannon Index | Phylogenetic Diversity | Richness | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Factor | R2 | P value | R2 | P value | Rho | P value | Rho | P value | Rho | P value |
| KCAL | 0.027 | 0.0433 | 0.025 | 0.0050 | 0.041 | 0.7260 | −0.092 | 0.4317 | −0.036 | 0.7587 |
| Protein | 0.015 | 0.3076 | 0.022 | 0.0261 | 0.176 | 0.1311 | 0.010 | 0.9290 | 0.052 | 0.6602 |
| %KCAL from protein | 0.017 | 0.2309 | 0.013 | 0.4127 | 0.157 | 0.1794 | 0.158 | 0.1754 | 0.137 | 0.2424 |
| Fat | 0.026 | 0.0642 | 0.021 | 0.0267 | −0.022 | 0.8492 | −0.105 | 0.3712 | −0.070 | 0.5516 |
| %KCAL from fat | 0.012 | 0.4957 | 0.015 | 0.2329 | −0.084 | 0.4726 | −0.077 | 0.5125 | −0.092 | 0.4335 |
| Carbohydrate | 0.024 | 0.0782 | 0.023 | 0.0167 | 0.068 | 0.5604 | −0.104 | 0.3723 | −0.021 | 0.8595 |
| %KCAL from carb. | 0.011 | 0.6011 | 0.011 | 0.8244 | −0.041 | 0.7277 | −0.043 | 0.7160 | −0.012 | 0.9206 |
| Fiber | 0.014 | 0.3613 | 0.015 | 0.2167 | 0.039 | 0.7415 | 0.074 | 0.5279 | 0.062 | 0.5950 |
| Calcium | 0.010 | 0.6201 | 0.013 | 0.5040 | −0.088 | 0.4541 | −0.134 | 0.2505 | −0.207 | 0.0744 |
| Copper | 0.017 | 0.2294 | 0.022 | 0.0217 | −0.183 | 0.1171 | −0.137 | 0.2396 | −0.145 | 0.2131 |
| Iron | 0.030 | 0.0278 | 0.019 | 0.0725 | 0.181 | 0.1211 | 0.089 | 0.4495 | 0.145 | 0.2160 |
| Magnesium | 0.016 | 0.2882 | 0.019 | 0.0704 | −0.162 | 0.1657 | −0.090 | 0.4436 | −0.130 | 0.2655 |
| Phosphorous | 0.008 | 0.7882 | 0.016 | 0.206 | −0.034 | 0.7709 | −0.090 | 0.4442 | −0.120 | 0.3036 |
| Potassium | 0.018 | 0.1859 | 0.018 | 0.0860 | −0.035 | 0.7681 | −0.059 | 0.6149 | −0.069 | 0.5586 |
| Selenium | 0.019 | 0.1825 | 0.030 | 0.001 | 0.282 | 0.0141 | 0.068 | 0.5617 | 0.139 | 0.2345 |
| Sodium | 0.039 | 0.0057 | 0.031 | 0.0004 | 0.106 | 0.3664 | −0.111 | 0.3423 | 0.004 | 0.9754 |
| Zinc | 0.033 | 0.0173 | 0.017 | 0.1509 | 0.059 | 0.6137 | −0.015 | 0.8965 | 0.007 | 0.9558 |
| Vitamin A (RAEa) | 0.012 | 0.512 | 0.011 | 0.7476 | 0.070 | 0.5507 | 0.007 | 0.9533 | −0.020 | 0.8625 |
| Thiamin (B1) | 0.038 | 0.0081 | 0.023 | 0.0156 | 0.141 | 0.2266 | 0.044 | 0.7088 | 0.070 | 0.5517 |
| Riboflavin (B2) | 0.021 | 0.1199 | 0.018 | 0.1017 | 0.056 | 0.6312 | 0.010 | 0.9303 | −0.021 | 0.8553 |
| Niacin (B3) | 0.028 | 0.0427 | 0.026 | 0.0037 | 0.242 | 0.0367 | 0.078 | 0.5082 | 0.141 | 0.2265 |
| Pyridoxine (B6) | 0.023 | 0.0913 | 0.020 | 0.0381 | 0.154 | 0.1882 | 0.017 | 0.8842 | 0.065 | 0.5803 |
| Folate (B9) | 0.024 | 0.0729 | 0.021 | 0.0277 | 0.195 | 0.0930 | 0.047 | 0.6905 | 0.101 | 0.3877 |
| Vitamin B12 | 0.007 | 0.8135 | 0.013 | 0.5043 | 0.202 | 0.0815 | 0.085 | 0.4673 | 0.098 | 0.4010 |
| Vitamin C | 0.008 | 0.7752 | 0.017 | 0.1340 | 0.137 | 0.2398 | −0.102 | 0.3844 | 0.044 | 0.7081 |
| Vitamin D | 0.012 | 0.4989 | 0.014 | 0.4098 | −0.005 | 0.9664 | 0.075 | 0.5224 | −0.030 | 0.7992 |
| Vitamin E | 0.010 | 0.6308 | 0.016 | 0.1887 | 0.029 | 0.8031 | 0.011 | 0.9220 | 0.018 | 0.8765 |
| Vitamin K | 0.011 | 0.5990 | 0.013 | 0.5342 | 0.026 | 0.8272 | −0.068 | 0.5600 | −0.022 | 0.8545 |
| Cholesterol | 0.013 | 0.4381 | 0.016 | 0.1766 | 0.164 | 0.1592 | 0.186 | 0.1107 | 0.174 | 0.1347 |
| Saturated FA | 0.019 | 0.1700 | 0.015 | 0.2663 | 0.047 | 0.6862 | −0.061 | 0.6027 | −0.057 | 0.6272 |
| Monounsaturated FA | 0.023 | 0.0920 | 0.021 | 0.0362 | −0.060 | 0.6098 | −0.104 | 0.3723 | −0.083 | 0.4804 |
| Polyunsaturated FA | 0.020 | 0.1436 | 0.022 | 0.0217 | −0.079 | 0.5006 | −0.124 | 0.2907 | −0.076 | 0.5157 |
| Choline | 0.010 | 0.6771 | 0.015 | 0.2833 | 0.120 | 0.3041 | 0.121 | 0.2995 | 0.116 | 0.3198 |
RAE: retinol activity equivalents.
Beta diversity
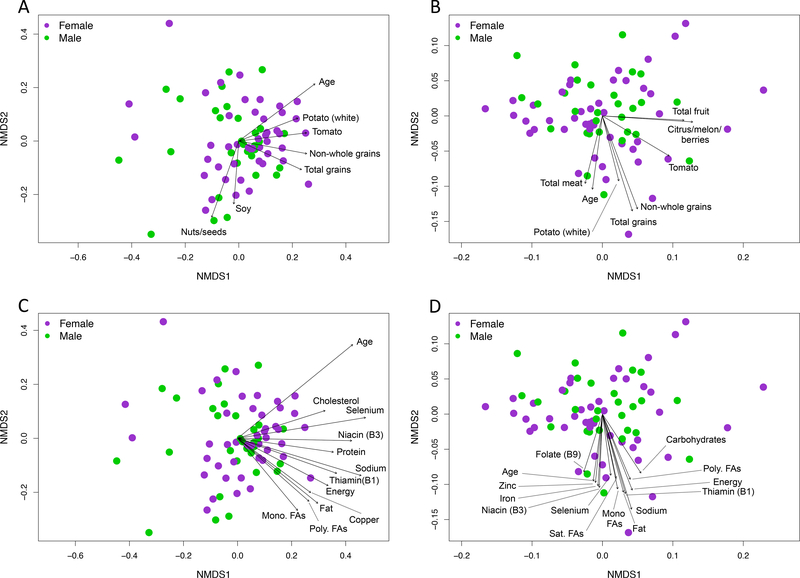
Similar to what was observed with alpha diversity, age was strongly associated with microbiome beta diversity while sex was not (Table 3). Food groups that were significantly associated with microbiome structure (weighted UniFrac) included total fruit, total grain, and plant protein consumption (Table 3). Additionally, total grain and total vegetable consumption were significantly associated with community membership (unweighted UniFrac). For both community structure and membership, consumption of non-whole grains was strongly associated with the variation observed in the grain food group. Tomato consumption was also strongly related to the variation with both beta diversity metrics. Yogurt, soy, and nut/seed consumption was associated with variation only for community membership. Using the ‘envfit’ function in the R package vegan, many of these same food groups were significantly correlated with axes coordinates illustrating the directional pull of these factors in ordination space (Figure 1A & B). With regards to macronutrients, total protein, fat, and carbohydrate consumption were related to community membership (Table 4). Several micronutrients, including a number of minerals and B vitamins were also associated with community structure and membership (Table 4, Figure 1). The most likely dietary sources of B-vitamins and minerals include fortified, non-whole grains 286 and meats (Figure 2).
Figure 1.
Consumption of specific food groups (A & B) and nutrients (C & D) were strongly associated with gut microbiome membership (unweighted UniFrac, A & C) and structure (weighted UniFrac, B & D) in children aged 2–9 years. Each point in each non-metric multidimensional scaling (NMDS) plot represents the average microbiome composition of one child across three time points and are colored based on sex of the child. Vectors depict significant correlations (P ≤ 0.05) between specific dietary features and microbiome composition across the study population as determined from 10,000 permutations using the ‘envfit’ function in R vegan package. Text files of ‘envfit’ results are available online at figshare34.
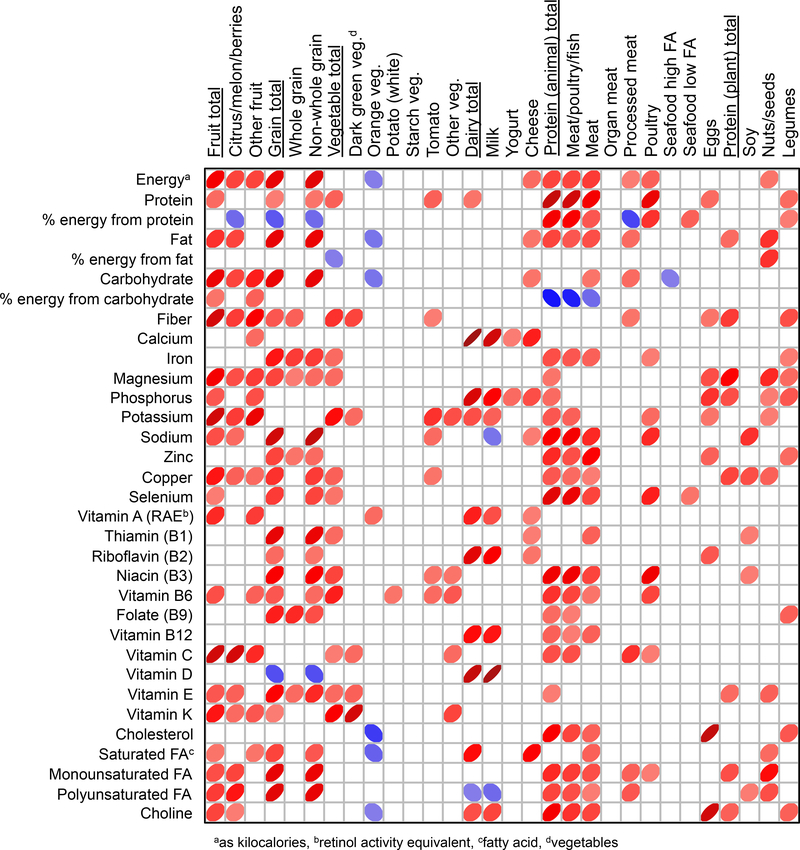
Figure 2.
Spearman rank correlation matrix showing relationships between food group consumption and average nutrient intake in the study population. Red colors illustrate positive correlations while blue illustrate negative correlations. The width of each ellipse and shade of color is proportional to each Rho value with wider widths and lighter color shade indicating lower Rho values. Only correlations with P-values < 0.05 are shown.
Taxonomy
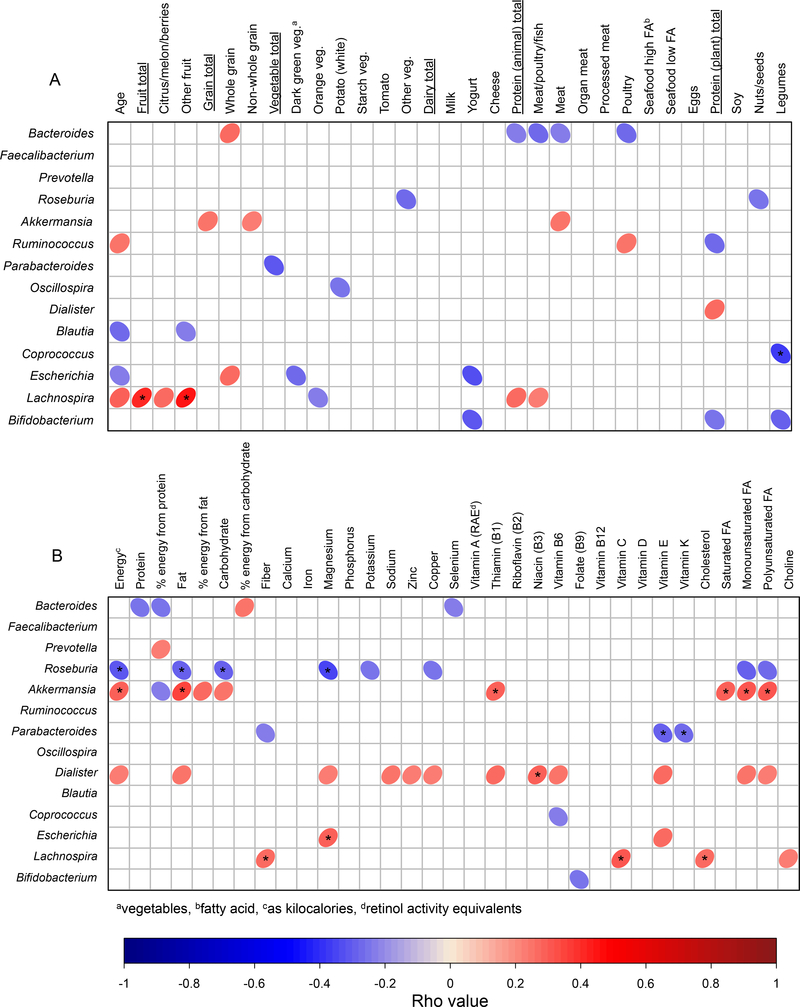
A few genus-level taxa including Ruminococcus (positive) and Blautia (negative) were correlated with age (Figure 3A). Likewise, several genera were correlated with the consumption of specific food groups and subcategories (Figure 3A). For example, Bacteroides, the most abundant genera observed on average (Figure 4), was positively correlated with the amount of whole grains and negatively correlated with the amount of animal protein consumed. Many of the same macronutrients and micronutrients associated with microbiome beta diversity were also correlated with the abundance of several taxonomic groups (Figure 3B).
Figure 3.
The relative abundance of several bacterial genera were correlated with consumption of several food groups (A) and with nutrient intake (B) in a diverse population of children aged 2–9 years. Age (A) was also correlated with several bacterial genera. Only correlations with uncorrected P-values less than 0.05 are shown as ellipses with asterisks indicating significance after FDR correction (corrected P ≤ 0.05). Red ellipses indicate a positive association, whereas blue ellipses indicate a negative correlation. The width of each ellipse is proportional to the strength of the correlation (i.e. Spearman Rho values) with narrower ellipse indicating a more significant relationship (i.e. a lower P-value). Darker colors of red and blue indicate Spearman Rho values closer to 1 and −1, respectively.
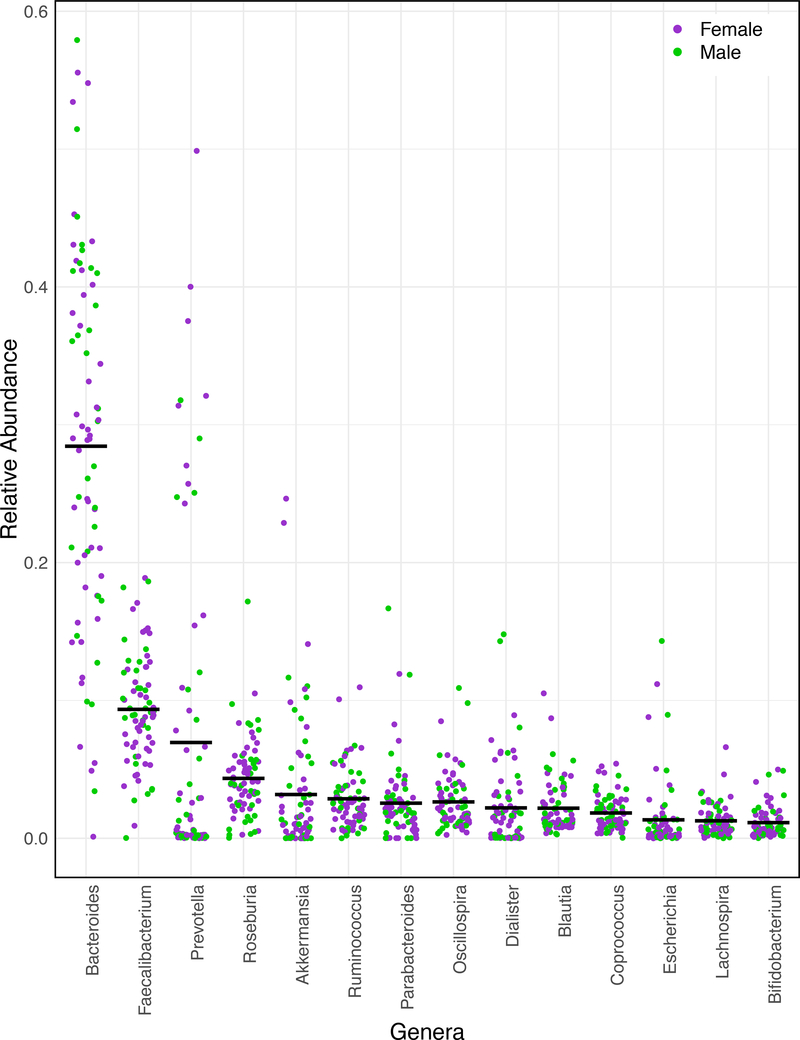
Figure 4.
Taxonomic composition of the child gut microbiome. Each point represents the average abundance of each genera across three time points for each of 75 children. Bars illustrate mean values for each genera across the population. Only genera greater that 1% on average are shown.
DISCUSSION
The goal of this study was to link quantitative dietary intake with gut microbiome features in a healthy population of post-weaned US children aged 2–9 years. Both diet and microbiome composition were averaged across three, non-consecutive time points of healthy subjects to approximate “recent intake” and minimize dietary and microbiome irregularities. This was the method used in this study to identify linkages between dietary intake and microbiome features in an understudied US demographic.
Other studies among young children assessing the relationship between the gut microbiome and dietary intake have used both 24-hour dietary recall information over three consecutive days62 or 3-day food records17 in addition to food frequency questionnaires, but in these studies assessment of dietary intake took place over longer time periods from six months up to one year. The present study was conducted over the summer months and thus, averaging three days of non-consecutive intake allowed for the estimation of recent dietary intake to be correlated with the fecal samples that were collected during the same time period.
The dietary intake of this study population is similar in some ways to the nation as a whole, and different in others. In a nationally representative study of children ages 2–11 years using a combined data set from What We Eat in America (WWEIA), NHANES, and the School Nutrition Dietary Assessment Study (SNDA), the authors demonstrated low intakes of vitamin D, calcium, and potassium and excess intakes for energy, carbohydrates and sodium63. In contrast, the population studied here had intakes of calcium at or just above the EAR. Hamner et al64 examined mineral intakes for two-year old children in NHANES between 2003–2012. They showed that 2% or fewer had usual intakes below the EAR for iron, calcium, and zinc with one in every two children exceeding the upper limit (UL) for zinc. Similarly, 36% of all participants in this study exceeded the UL for zinc and although intakes of iron exceeded the EAR by more than 3 times the requirement for 54.7% of participants, no one exceeded the UL for iron intake. Results from the recent Feeding Infant and Toddlers Study (FITS), which assessed dietary practices of children in the US from birth to 48 months demonstrated low intakes of potassium, fiber, vitamin D, and vitamin E, and high intakes of zinc, sodium, and saturated fats – similar to the results for this study65. When examining the AMDRs for macronutrient intake, low proportions of study participants demonstrated excesses. While 28% of study participants had a value lower than the AMDR for total fat, 82.7% had saturated fat intake greater than the AMDR of < 10%. This means that while total fat intake may have been low for about one-third of study participants, the quality of their fat intake was poor with many of them consuming animal fats as their primary source. Almost 91% of study participants had fiber intakes below recommendations as well. Berding et al17, also showed lower intakes for fiber and high intakes of saturated fat in their study of children 4–8 years when associating dietary patterns with microbiome composition. National intakes of protein for children 2–3 years and 4–8 years show average intakes around 14%–16%, which tracks with the population in this study66. Sodium intake exceeded the upper limit (UL) for 78.7% of study participants60. This observation is similar to national studies that show children in the US exceed recommended intakes of sodium, but have low intakes of fiber67,68. This may also explain in part the high sodium intake as many of the protein-rich foods included hot dogs, chicken nuggets and pizza. Finally, intakes of B-vitamins were high and exceeded ULs for niacin, vitamin B6, and folate. One reason for this may be a high intake of ready-to-eat (RTE) cereals. Over 46% of the families reported annual incomes less than $30,000 and 53.3% participated in the Special Supplemental Nutrition Program for WIC. RTE cereals and whole grain bread are components of the WIC food package, which may allow greater access than usual for the families in our study to these food items, which are often fortified with B-vitamins.
One of the strongest factors related to microbiome features observed was a child’s age. Older children tended to have more diverse and more even gut communities than younger children suggesting that assembly of the gut microbiome continues well into childhood. Likewise, community membership and structure were associated with age. Previous studies relying on high throughput molecular techniques have suggested that by about the age of 3, a child’s microbiome reaches diversity levels proximate to that of an adult13,15. Other more recent studies have observed continued development of the gut microbiome beyond three years of age, similar to what was observed here17,69. From a human development perspective, early childhood represents a period of time when children are beginning to explore greater dietary diversity. They are becoming more independent in their eating habits and shifting from a higher fat diet to one that has a greater proportion of carbohydrates and protein70. As children approach middle childhood, they continue to gain independence in their eating habits and may be more likely to purchase food on their own from school, corner stores, and fast food outlets, further expanding their dietary diversity. With guidance from adults, this can be a stage when children learn healthy habits and continue to expand the numbers and types of healthy foods they consume to ensure healthy weight gain and prevention of later chronic diseases70. Taken together, results presented here demonstrate continued development of the gut microbiome beyond the age of 3 and because this is also an important developmental stage for humans, its hypothesized that early and middle childhood may represent a crucial window when the gut microbiome may still be amenable to lasting manipulations through diet.
Consumption of several food groups were associated with different microbiome features. Interestingly, with one exception (i.e. yogurt), animal-derived foods were not strongly associated with microbiome structure or membership. Instead, protein-rich animal products like meats were positively correlated with microbial diversity (as Shannon Index). When total protein consumption as a macronutrient was examined, there was no correlation with any of the alpha diversity metrics, likely because this category includes all dietary sources of proteins regardless of whether they were plant or animal derived. Several previous studies have observed increases in microbiome diversity related to increased consumption of proteins32,71–75. However, people in industrialized nations consuming a diet rich in animal proteins and fats but poor in plant-based fibers (i.e. a ‘Western diet’), generally have less diverse gut communities than people eating predominantly plant-based diets13,32,76,77. Results observed here suggest that within populations consuming a Western diet, the source of proteins consumed, whether plant or animal based, may also be an important determinant of microbiome diversity.
Among the plant-derived foods, grain consumption, specifically non-whole grains, was one of the strongest factors associated with microbiome structure and membership. Grains, including non-whole grains, contain a mixture of indigestible starches and fibers that pass through the small intestine to the large intestine where they are fermented into short-chain fatty acids (SCFA) by various members of the gut microbiome78. Non-whole grain foods like breakfast cereals, loaf breads, and pasta, are often enriched with several B-vitamins (e.g. thiamin, riboflavin, and niacin) and minerals (e.g. iron) that are lost during processing. Many of these same micronutrients were also strongly associated with microbiome structure and composition. Data from a recent study of the major contributors to energy intake of children 2 – 18 years in the past 21 years shows that pizza, grain-based desserts, breads, pasta and ready to eat (RTE) cereals were among the top ten contributors79. These foods were also among the top contributors to diets of the children studied here. Many of these grain-based products are fortified with B-vitamins and may explain the high intakes observed. Dietary micronutrients are also known to be associated with the composition of the gut microbiome when deficient80–82. However, their relationship with the gut microbiome when consumed in excess is less understood. Whether the differences in microbiome features observed are related to the indigestible starches and fibers or the added micronutrients in these grain foods is unknown but they do provide intriguing targets for future interventions or model organism-based studies. B-vitamins in particular are known to mediate numerous symbiotic interactions between eukaryotes and prokaryotes in a number of systems83–85. For example, vitamin B12 has been suggested to be a modulator of gut microbial ecology86.
Other plant-based foods that were significantly associated with microbiome composition included fruits. Within fruits, the category that includes citrus, melons, and berries was associated with much of the variation in microbiome structure, but not membership. These fruits are significant sources of fiber, pectin, and polyphenols, dietary compounds that are known to be associated with the diversity of gut bacteria87–93. The only genus that was positively associated with fruit consumption here was Lachnospira, a member of the Firmicutes phylum that is known to contain pectin fermenting species94. Other human observational studies have also found positive associations between Lachnospira abundance and consumption of fruits95 and vegetables62. Decreases in the relative abundance of members of the Lachnospira lineage have been observed in asthmatic children96 and adults with gallstones97 or HIV98, suggesting some sort of beneficial role for this lineage. Future research should more directly address how members of the Lachnospira lineage interact with its human host.
Although an extremely diverse phylum, human associated Bacteroidetes are considered ecological generalists able to consume a diversity of dietary and host-produced substrates99. On average, the two most abundant genera within the Bacteroidetes observed were Bacteroides and Prevotella. Theses genera are common members of the gut microbiome in a diversity of populations and their abundances have been linked to broad scale dietary habits in several previous studies. For example, Prevotella are enriched in populations consuming fiber-rich diets like those in rural Africa and South America, whereas the Bacteroides are typically enriched in individuals consuming a Western diet13,32,77. The abundance of Prevotella is also linked to a vegetarian diet in Western populations19. In this study, children who consumed more whole grains and fewer meat products were enriched in Bacteroides while Prevotella were not associated with consumption of any food groups. Both of these observations are in contrast to most previous studies as stated above. Linking these genera to more fine-scale dietary components, however, has proven difficult likely because of diversity at the species and strain levels as recently investigated by De Filippis and colleagues100. They found sub-genus oligotypes of Prevotella and Bacteroides that were both associated with plant-based and animal-based diets, suggesting the current paradigm regarding the abundances of these genera (i.e agrarian/vegetarian vs. meat-based diets) may be an oversimplification. It is worth noting, however, that in isolation, species of these genera do have strikingly different responses to bile salts with Prevotella spp. generally being more sensitive than Bacteroides spp.101. More fine-scale taxonomic resolution coupled with laboratory culturing experiments may be needed to better define the niche space of these common gut bacteria.
While the current study has identified dietary factors that are associated with features of the microbiome this study does have limitations. Sample size is perhaps the most obvious limitation of this study as it has been suggested that more than 40,000 humans would be needed to observe the full diversity of the gut microbiome20. From a microbiome perspective, this study has only examined taxonomic differences and inferred how this may be related to the function of these communities. A multi-omic approach that includes metagenomics, transcriptomics, and metabolomics would be able to better elucidate more direct relationship between diet and the microbiome. Furthermore, these correlative results should be interpreted with caution, as correlations do not determine causation. More controlled studies involving animal models may be able to more directly assess the linkages uncovered in this study. Ideally, dietary assessment would have included both a 3-day food record and a food frequency questionnaire to better estimate dietary habits over a longer period of time and to understand how the dietary data collected reflect typical dietary intake. However, given that study participants were recruited during the summer months in a community-based setting and parents were the primary caregivers during the time period of data collection and were with children for meals, averaging the three, non-consecutive 24-hour multiple pass dietary recalls that included weekdays and weekend days and using parents as proxy reporters was considered the most appropriate method to estimate both energy and nutrient intakes of these children. However, it is also important to acknowledge potential for bias associated with self-report of dietary intake. Overreporting and underreporting of dietary intake using parents as proxies for the age group under study is a common concern and has been documented102. Comparisons of parental estimates of energy intake compared to DLW have been found to be in congruence at the group level, but individual level estimates often reveal under- or overreporting 103,104. Desirability bias may also cause parents to alter the foods they provide their children to make it appear they are offering healthier options when recording dietary intake105.
In addition, while age is an important characteristic that may be key in better understanding the relationship between dietary intake and the gut microbiome, stratifying by age within this population to compare younger and older children would have limited the power of the results. Future studies should include a larger number of participants to be able to tease apart both the developmental differences that may be occurring in the microbiome due to changes in eating habits as well as to better structure potential interventions to ensure healthy eating habits at the earliest stage possible.
CONCLUSION
Findings presented here suggest the importance of diet in structuring overall gut microbiome composition throughout early life. By taking an interdisciplinary approach, this study was able to identify key dietary features that may have important roles in structuring the child gut microbiome. In particular, the consumption of fortified, non-whole grain foods enriched in vitamins and minerals were among the strongest factors associated with gut microbiome features.
Research Snapshot.
Research Question
Is diet associated with the diversity and composition of the gut microbiome in a diverse population of American children?
Key Findings
In this cross-sectional study that included 75 children between the ages of 2–9 years, several food groups and nutrients were linked to differences in gut microbiome composition. For example, consumption of non-whole grain foods and some B-vitamins were significantly linked to community structure and membership. A variety of bacterial taxa were also significantly correlated with consumption of particular food groups or nutrients.
Acknowledgments
The authors would like to thank Joan Maltese, Zelzah Guzman, Alison Gambou, Ann -Marie Pham, Griseida Ruiz, and Jessica Saavedra for all their efforts in participant recruitment.
We have received permission of the individuals named to include them in our acknowledgements section.
Funding: This work was supported by the NIH National Institute of General Medical Sciences (RL5GM118975), the Department of Biology at California State University, Northridge (CSUN), the CSUN Research, Scholarship and Creative Activity Award, and the Suzan Jean Lebowitz Research Fund.
Footnotes
Conflict of interest disclosures: The authors of the manuscript have no conflicts of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Dena Herman, Professor, Department of Family and Consumer Sciences, California State University, Northridge, California, USA, 18111 Nordhoff Street, Northridge, CA, 91330.
Nicholas Rhoades, Graduate Student, Department of Biology, California State University, Northridge, California, USA, 18111 Nordhoff Street, Northridge, CA, 91330.
Jasmine Mercado, Department of Family and Consumer Sciences, California State University, Northridge, 18111; Nordhoff Street, Northridge, CA, 91330.
Pedro Argueta, Department of Family and Consumer Sciences, California State University, Northridge, 18111 Nordhoff Street, Northridge, CA, 91330.
Ulises Lopez, Graduate Student, Department of Biology, California State University, Northridge, California, USA, 18111 Nordhoff Street, Northridge, CA, 91330.
Gilberto E. Flores, Assistant Professor, Department of Biology, California State University, Northridge, California, USA, 18111 Nordhoff Street, Northridge, CA, 91330.
References
- 1.Qin J, Li R, Raes J, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464(7285):59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Young VB. The intestinal microbiota in health and disease. Current opinion in gastroenterology. 2012;28(1):63–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maynard CL, Elson CO, Hatton RD, Weaver CT. Reciprocal interactions of the intestinal microbiota and immune system. Nature. 2012;489(7415):231–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Costello EK, Lauber CL, Hamady M, Fierer N, Gordon JI, Knight R. Bacterial community variation in human body habitats across space and time. Science. 2009;326(5960):1694–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flores GE, Caporaso JG, Henley JB, et al. Temporal variability is a personalized feature of the human microbiome. Genome biology. 2014;15(12):531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Turnbaugh PJ, Ridaura VK, Faith JJ, Rey FE, Knight R, Gordon JI. The effect of diet on the human gut microbiome: a metagenomic analysis in humanized gnotobiotic mice. Sci Transl Med. 2009;1(6):6ra14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spor A, Koren O, Ley R. Unravelling the effects of the environment and host genotype on the gut microbiome. Nature reviews. Microbiology. 2011;9(4):279–290. [DOI] [PubMed] [Google Scholar]
- 8.Cabrera-Rubio R, Collado MC, Laitinen K, Salminen S, Isolauri E, Mira A. The human milk microbiome changes over lactation and is shaped by maternal weight and mode of delivery. Am J Clin Nutr. 2012;96(3):544–551. [DOI] [PubMed] [Google Scholar]
- 9.Martin R, Makino H, Cetinyurek Yavuz A, et al. Early-Life Events, Including Mode of Delivery and Type of Feeding, Siblings and Gender, Shape the Developing Gut Microbiota. PLoS One. 2016;11(6):e0158498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Le Huërou-Luron I, Blat S, Boudry G. Breast- v. formula-feeding: impacts on the digestive tract and immediate and long-term health effects. Nutr Res Rev. 2010;23(1):23–36. [DOI] [PubMed] [Google Scholar]
- 11.Koenig JE, Spor A, Scalfone N, et al. Succession of microbial consortia in the developing infant gut microbiome. Proc Natl Acad Sci U S A. 2011;108 Suppl 1:4578–4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marques TM, Wall R, Ross RP, Fitzgerald GF, Ryan CA, Stanton C. Programming infant gut microbiota: influence of dietary and environmental factors. Curr Opin Biotechnol. 2010;21(2):149–156. [DOI] [PubMed] [Google Scholar]
- 13.Yatsunenko T, Rey FE, Manary MJ, et al. Human gut microbiome viewed across age and geography. Nature. 2012;486(7402):222–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ringel-Kulka T, Cheng J, Ringel Y, et al. Intestinal microbiota in healthy U.S. young children and adults--a high throughput microarray analysis. Plos One. 2013;8(5):e64315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arrieta MC, Stiemsma LT, Amenyogbe N, Brown EM, Finlay B. The intestinal microbiome in early life: health and disease. Frontiers in immunology. 2014;5:427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koenig JE, Spor A, Scalfone N, et al. Succession of microbial consortia in the developing infant gut microbiome. Proceedings of the National Academy of Sciences of the United States of America. 2011;108 Suppl 1:4578–4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berding K, Holscher HD, Arthur AE, Donovan SM. Fecal microbiome composition and stability in 4- to 8-year old children is associated with dietary patterns and nutrient intake. J Nutr Biochem. 2018;56:165–174. [DOI] [PubMed] [Google Scholar]
- 18.David LA, Maurice CF, Carmody RN, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505(7484):559–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu GD, Chen J, Hoffmann C, et al. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334(6052):105–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhernakova A, Kurilshikov A, Bonder MJ, et al. Population-based metagenomics analysis reveals markers for gut microbiome composition and diversity. Science. 2016;352(6285):565–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cordain L, Eaton SB, Sebastian A, et al. Origins and evolution of the Western diet: health implications for the 21st century. Am J Clin Nutr. 2005;81(2):341–354. [DOI] [PubMed] [Google Scholar]
- 22.Tsujimoto T, Kajio H, Sugiyama T. Obesity, diabetes, and length of time in the United States: Analysis of National Health and Nutrition Examination Survey 1999 to 2012. Medicine (Baltimore). 2016;95(35):e4578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart Disease and Stroke Statistics—2016 Update. Circulation. 2016;133(4):e38. [DOI] [PubMed] [Google Scholar]
- 24.National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States, Atlanta, GA: US Department of Health and Human Services; 2014; 2014. [Google Scholar]
- 25.Peery AF, Dellon ES, Lund J, et al. Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology. 2012;143(5):1179–1187.e1171–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karlsson FH, Tremaroli V, Nookaew I, et al. Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature. 2013;498(7452):99–103. [DOI] [PubMed] [Google Scholar]
- 27.West CE, Renz H, Jenmalm MC, et al. The gut microbiota and inflammatory noncommunicable diseases: Associations and potentials for gut microbiota therapies. Journal of Allergy and Clinical Immunology. 2015;135(1):3–14. [DOI] [PubMed] [Google Scholar]
- 28.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesityassociated gut microbiome with increased capacity for energy harvest. Nature. 2006;444(7122):1027–1031. [DOI] [PubMed] [Google Scholar]
- 29.Chassard C, Dapoigny M, Scott KP, et al. Functional dysbiosis within the gut microbiota of patients with constipated-irritable bowel syndrome. Alimentary pharmacology & therapeutics. 2012;35(7):828–838. [DOI] [PubMed] [Google Scholar]
- 30.Tremaroli V, Backhed F. Functional interactions between the gut microbiota and host metabolism. Nature. 2012;489(7415):242–249. [DOI] [PubMed] [Google Scholar]
- 31.Heilbronn LK, Campbell LV. Adipose tissue macrophages, low grade inflammation and insulin resistance in human obesity. Current pharmaceutical design. 2008;14(12):1225–1230. [DOI] [PubMed] [Google Scholar]
- 32.De Filippo C, Cavalieri D, Di Paola M, et al. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(33):14691–14696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yatsunenko T, Rey FE, Manary MJ, et al. Human gut microbiome viewed across age and geography. Nature. 2012;486(7402):222–+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.[dataset] Herman D, Flores GE. Sequence and supplementary data for “ Dietary habits of two to nine-year-old American children are associated with gut microbiome composition.”. Figshare, v3; 2019. 10.6084/m9.figshare.7011272.v3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gonzalez A, Navas-Molina JA, Kosciolek T, et al. Qiita: rapid, web-enabled microbiome meta-analysis. Nat Methods. 2018;15(10):796–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.[dataset] Herman D, Flores GE. Sequence Data for “ Dietary habits of two to nine-year-old American children are associated with gut microbiome composition. Qiita, Study 12360; 2019. https://qiita.ucsd.edu/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Song SJ, Lauber C, Costello EK, et al. Cohabiting family members share microbiota with one another and with their dogs. Elife. 2013;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Caporaso JG, Lauber CL, Costello EK, et al. Moving pictures of the human microbiome. Genome biology. 2011;12(5):R50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Caporaso JG, Lauber CL, Walters WA, et al. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proceedings of the National Academy of Sciences of the United States of America. 2011;108 Suppl 1:4516–4522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Edgar RC. UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nature methods. 2013;10(10):996–998. [DOI] [PubMed] [Google Scholar]
- 41.Wang Q, Garrity GM, Tiedje JM, Cole JR. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Applied and Environmental Microbiology. 2007;73(16):5261–5267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.DeSantis TZ, Hugenholtz P, Larsen N, et al. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Applied and Environmental Microbiology. 2006;72(7):5069–5072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McDonald D, Price MN, Goodrich J, et al. An improved Greengenes taxonomy with explicit ranks for ecological and evolutionary analyses of bacteria and archaea. The ISME journal. 2012;6(3):610–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Caporaso JG, Kuczynski J, Stombaugh J, et al. QIIME allows analysis of high-throughput community sequencing data. Nature methods. 2010;7(5):335–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Price MN, Dehal PS, Arkin AP. FastTree: computing large minimum evolution trees with profiles instead of a distance matrix. Molecular biology and evolution. 2009;26(7):1641–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Faith DP. Conservation Evaluation and Phylogenetic Diversity. Biological Conservation. 1992;61(1):1–10. [Google Scholar]
- 47.Lozupone C, Knight R. UniFrac: a new phylogenetic method for comparing microbial communities. Applied and Environmental Microbiology. 2005;71(12):8228–8235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Burrows TL, Martin RJ, Collins CE. A systematic review of the validity of dietary assessment methods in children when compared with the method of doubly labeled water. J Am Diet Assoc. 2010;110(10):1501–1510. [DOI] [PubMed] [Google Scholar]
- 49.Johnson RK, Driscoll P, Goran MI. Comparison of multiple-pass 24-hour recall estimates of energy intake with total energy expenditure determined by the doubly labeled water method in young children. J Am Diet Assoc. 1996;96(11):1140–1144. [DOI] [PubMed] [Google Scholar]
- 50.Moshfegh AJ, Rhodes DG, Baer DJ, et al. The US Department of Agriculture Automated Multiple-Pass Method reduces bias in the collection of energy intakes. The American journal of clinical nutrition. 2008;88(2):324–332. [DOI] [PubMed] [Google Scholar]
- 51.National Cancer Institute. Automated Self-Administered 24-Hour (ASA24) Dietary Assessment Tool. 2019; Available at https://epi.grants.cancer.gov/asa24/ Accessed May 2019.
- 52.U.S. Departments of Health and Human Services and U.S. Department of Agriculture. 2015–2020 Dietary Guidelines for Americans; 2015; Available at, https://health.gov/dietaryguidelines/2015/guidelines/ 8th edtion. [Google Scholar]
- 53.Bowman SA, Friday JE, and Moshfegh AJ. MyPyramid Equivalents Database, 2.0 for USDA Survey Foods, 2003–2004. U.S. Department of Agriculture, ARS; 2008. http://www.ars.usda.gov/ba/bhnrc/fsrg. [Google Scholar]
- 54.Center for Nutrtion Policy and Promotion & United States Department of Agriculture. Healthy Eating Index Support Files 07 08. http://www.cnpp.usda.gov/healthy-eating-index-support-files-07-08 Accessed May 2019.
- 55.R: A language and environment for statistical computing [computer program]. Vienna, Austria: R Foundation for Statistical Computing; 2013. [Google Scholar]
- 56.psych: Procedures for Personality and Psychological Research [computer program]. Version 1.7.5. https://CRAN.R-project.org/package=psych2017. [Google Scholar]
- 57.corrplot: Visualization of a Correlation Matrix [computer program]. Version 0.77. https://CRAN.R-project.org/package=corrplot2016.
- 58.vegan: Community Ecology Package [computer program]. https://CRAN.R-project.org/package=vegan2017.
- 59.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society. Series B (Methodological). 1995;57(1):289–300. [Google Scholar]
- 60.Institute of Medicine. Dietary Reference Intakes: The Essential Guide to Nutrient Requirements. Washington, DC: The National Academies Press; 2006. [Google Scholar]
- 61.Trumbo P, Schlicker S, Yates AA, Poos M, Food, Nutrition Board of the Institute of Medicine TNA. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein and amino acids. J Am Diet Assoc. 2002;102(11):1621–1630. [DOI] [PubMed] [Google Scholar]
- 62.Smith-Brown P, Morrison M, Krause L, Davies PS. Dairy and plant based food intakes are associated with altered faecal microbiota in 2 to 3 year old Australian children. Sci Rep. 2016;6:32385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hess J, Slavin J. Snacking for a cause: nutritional insufficiencies and excesses of U.S. children, a critical review of food consumption patterns and macronutrient and micronutrient intake of U.S. children. Nutrients. 2014;6(11):4750–4759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hamner HC, Perrine CG, Scanlon KS. Usual Intake of Key Minerals among Children in the Second Year of Life, NHANES 2003–2012. Nutrients. 2016;8(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bailey RL, Catellier DJ, Jun S, et al. Total Usual Nutrient Intakes of US Children (Under 48 Months): Findings from the Feeding Infants and Toddlers Study (FITS) 2016. J Nutr. 2018;148(9S):1557S–1566S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Berryman CE, Lieberman HR, Fulgoni VL 3rd, Pasiakos SM. Protein intake trends and conformity with the Dietary Reference Intakes in the United States: analysis of the National Health and Nutrition Examination Survey, 2001–2014. Am J Clin Nutr. 2018;108(2):405–413. [DOI] [PubMed] [Google Scholar]
- 67.Banfield EC, Liu Y, Davis JS, Chang S, Frazier-Wood AC. Poor Adherence to US Dietary Guidelines for Children and Adolescents in the National Health and Nutrition Examination Survey Population. J Acad Nutr Diet. 2016;116(1):21–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McGill CR, Fulgoni VL 3rd, Devareddy L. Ten-year trends in fiber and whole grain intakes and food sources for the United States population: National Health and Nutrition Examination Survey 2001–2010. Nutrients. 2015;7(2):1119–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hollister EB, Riehle K, Luna RA, et al. Structure and function of the healthy preadolescent pediatric gut microbiome. Microbiome. 2015;3:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Story M, Holt K, Sofka D. Bright Futures in Practice: Nutrition. 2nd ed. Arlington, VA: National Center for Education in Maternal and Child Health; 2002. [Google Scholar]
- 71.Jantchou P, Morois S, Clavel-Chapelon F, Boutron-Ruault MC, Carbonnel F. Animal protein intake and risk of inflammatory bowel disease: The E3N prospective study. Am J Gastroenterol. 2010;105(10):2195–2201. [DOI] [PubMed] [Google Scholar]
- 72.Meddah AT, Yazourh A, Desmet I, Risbourg B, Verstraete W, Romond MB. The regulatory effects of whey retentate from bifidobacteria fermented milk on the microbiota of the Simulator of the Human Intestinal Microbial Ecosystem (SHIME). J Appl Microbiol. 2001;91(6):1110–1117. [DOI] [PubMed] [Google Scholar]
- 73.Romond MB, Ais A, Guillemot F, Bounouader R, Cortot A, Romond C. Cell-free whey from milk fermented with Bifidobacterium breve C50 used to modify the colonic microflora of healthy subjects. J Dairy Sci. 1998;81(5):1229–1235. [DOI] [PubMed] [Google Scholar]
- 74.Swiatecka D, Narbad A, Ridgway KP, Kostyra H. The study on the impact of glycated pea proteins on human intestinal bacteria. Int J Food Microbiol. 2011;145(1):267–272. [DOI] [PubMed] [Google Scholar]
- 75.Clarke SF, Murphy EF, O’Sullivan O, et al. Exercise and associated dietary extremes impact on gut microbial diversity. Gut. 2014;63(12):1913–1920. [DOI] [PubMed] [Google Scholar]
- 76.Nakayama J, Yamamoto A, Palermo-Conde LA, et al. Impact of Westernized Diet on Gut Microbiota in Children on Leyte Island. Front Microbiol. 2017;8:197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Clemente JC, Pehrsson EC, Blaser MJ, et al. The microbiome of uncontacted Amerindians. Science advances. 2015;1(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Topping DL, Clifton PM. Short-chain fatty acids and human colonic function: roles of resistant starch and nonstarch polysaccharides. Physiol Rev. 2001;81(3):1031–1064. [DOI] [PubMed] [Google Scholar]
- 79.Slining MM, Mathias KC, Popkin BM. Trends in food and beverage sources among US children and adolescents: 1989–2010. J Acad Nutr Diet. 2013;113(12):1683–1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Degnan PH, Taga ME, Goodman AL. Vitamin B12 as a modulator of gut microbial ecology. Cell Metab. 2014;20(5):769–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hibberd MC, Wu M, Rodionov DA, et al. The effects of micronutrient deficiencies on bacterial species from the human gut microbiota. Sci Transl Med. 2017;9(390). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Subramanian S, Huq S, Yatsunenko T, et al. Persistent gut microbiota immaturity in malnourished Bangladeshi children. Nature. 2014;510(7505):417–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Michalkova V, Benoit JB, Weiss BL, Attardo GM, Aksoy S. Vitamin B6 generated by obligate symbionts is critical for maintaining proline homeostasis and fecundity in tsetse flies. Appl Environ Microbiol. 2014;80(18):5844–5853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Croft MT, Lawrence AD, Raux-Deery E, Warren MJ, Smith AG. Algae acquire vitamin B12 through a symbiotic relationship with bacteria. Nature. 2005;438(7064):90–93. [DOI] [PubMed] [Google Scholar]
- 85.Guillen-Navarro K, Encarnacion S, Dunn MF. Biotin biosynthesis, transport and utilization in rhizobia. FEMS Microbiol Lett. 2005;246(2):159–165. [DOI] [PubMed] [Google Scholar]
- 86.Degnan PH, Taga ME, Goodman AL. Vitamin B12 as a modulator of gut microbial ecology. Cell Metab. 2014;20(5):769–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cuervo A, Valdes L, Salazar N, et al. Pilot study of diet and microbiota: interactive associations of fibers and polyphenols with human intestinal bacteria. J Agric Food Chem. 2014;62(23):5330–5336. [DOI] [PubMed] [Google Scholar]
- 88.Eid N, Enani S, Walton G, et al. The impact of date palm fruits and their component polyphenols, on gut microbial ecology, bacterial metabolites and colon cancer cell proliferation. J Nutr Sci. 2014;3:e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Queipo-Ortuno MI, Boto-Ordonez M, Murri M, et al. Influence of red wine polyphenols and ethanol on the gut microbiota ecology and biochemical biomarkers. Am J Clin Nutr. 2012;95(6):1323–1334. [DOI] [PubMed] [Google Scholar]
- 90.Carvalho-Wells AL, Helmolz K, Nodet C, et al. Determination of the in vivo prebiotic potential of a maize-based whole grain breakfast cereal: a human feeding study. Br J Nutr. 2010;104(9):1353–1356. [DOI] [PubMed] [Google Scholar]
- 91.Costabile A, Klinder A, Fava F, et al. Whole-grain wheat breakfast cereal has a prebiotic effect on the human gut microbiota: a double-blind, placebo-controlled, crossover study. Br J Nutr. 2008;99(1):110–120. [DOI] [PubMed] [Google Scholar]
- 92.Halmos EP, Christophersen CT, Bird AR, Shepherd SJ, Gibson PR, Muir JG. Diets that differ in their FODMAP content alter the colonic luminal microenvironment. Gut. 2015;64(1):93–100. [DOI] [PubMed] [Google Scholar]
- 93.Keim NL, Martin RJ. Dietary whole grain-microbiota interactions: insights into mechanisms for human health. Adv Nutr. 2014;5(5):556–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Duskova D, Marounek M. Fermentation of pectin and glucose, and activity of pectin-degrading enzymes in the rumen bacterium Lachnospira multiparus. Lett Appl Microbiol. 2001;33(2):159–163. [DOI] [PubMed] [Google Scholar]
- 95.De Filippis F, Pellegrini N, Vannini L, et al. High-level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut. 2016;65(11):1812–1821. [DOI] [PubMed] [Google Scholar]
- 96.Stiemsma LT, Arrieta MC, Dimitriu PA, et al. Shifts in Lachnospira and Clostridium sp. in the 3-month stool microbiome are associated with preschool age asthma. Clin Sci (Lond). 2016;130(23):2199–2207. [DOI] [PubMed] [Google Scholar]
- 97.Wu T, Zhang Z, Liu B, et al. Gut microbiota dysbiosis and bacterial community assembly associated with cholesterol gallstones in large-scale study. BMC Genomics. 2013;14:669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.McHardy IH, Li X, Tong M, et al. HIV Infection is associated with compositional and functional shifts in the rectal mucosal microbiota. Microbiome. 2013;1(1):26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Koropatkin NM, Cameron EA, Martens EC. How glycan metabolism shapes the human gut microbiota. Nature reviews. Microbiology. 2012;10(5):323–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.De Filippis F, Pellegrini N, Laghi L, Gobbetti M, Ercolini D. Unusual sub-genus associations of faecal Prevotella and Bacteroides with specific dietary patterns. Microbiome. 2016;4(1):57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shah HN, Collins DM. Prevotella, a new genus to include Bacteroides melaninogenicus and related species formerly classified in the genus Bacteroides. Int J Syst Bacteriol. 1990;40(2):205–208. [DOI] [PubMed] [Google Scholar]
- 102.Baranowski T, Sprague D, Baranowski JH, Harrison JA. Accuracy of maternal dietary recall for preschool children. J Am Diet Assoc. 1991;91(6):669–674. [PubMed] [Google Scholar]
- 103.Reilly JJ, Montgomery C, Jackson D, MacRitchie J, Armstrong J. Energy intake by multiple pass 24 h recall and total energy expenditure: a comparison in a representative sample of 3–4-year-olds. Br J Nutr. 2001;86(5):601–605. [DOI] [PubMed] [Google Scholar]
- 104.Bornhorst C, Bel-Serrat S, Pigeot I, et al. Validity of 24-h recalls in (pre-)school aged children: comparison of proxy-reported energy intakes with measured energy expenditure. Clin Nutr. 2014;33(1):79–84. [DOI] [PubMed] [Google Scholar]
- 105.Foster E, Bradley J. Methodological considerations and future insights for 24-hour dietary recall assessment in children. Nutr Res. 2018;51:1–11. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Raw sequence data, accompanying metadata, and all supplemental data are available online at figshare34. Sequence data are also available through Qiita35,36 (QIITA: 12360).