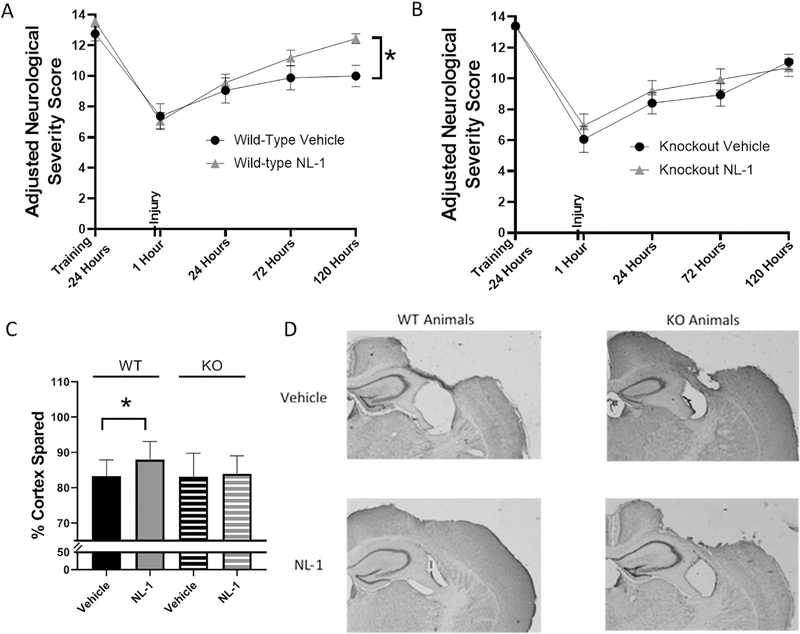
Figure 5: NL-1 produces significant functional recovery and neuroprotection, an effect lost in mitoNEET null mice.
Adult wild-type and mitoNEET null mice were trained on the beam walk test, the main component used for adjusted neurological severity score (aNSS), and then received a severe CCI injury 24 hours after training. Mice were given serial IP injections of NL-1 (10 mg/kg) at 15 mins and then every 24 hrs thereafter for the first 7 days post-injury. At 1 hr, 1 day, 3 days and 5 days post-injury, the beam walk test was performed and foot faults, inversions and ability to traverse the beams were measured and used to calculate aNSS (see Methods). (A) Wild-type mice treated with NL-1 had significantly improved performance at 5 days (120 h) post-injury compared to vehicle-treated mice. (B) There were no significant differences between NL-1 treatment and vehicle treatment in mitoNEET null mice. n=15–16/group, mean ± SEM, Two-way ANOVA, Compared Drug Treated to Vehicle within same genotype, Sidak Post-Hoc, F4,120 = 1.96, * p=60.025 (5 days post-injury) for wild-type animals; F4,116 = 0.48 for mitoNEET null animals. (C) Wild-type mice administered NL-1 (10 mg/kg) had a significant increase in cortical sparing (37%) compared to vehicle-treated mice at 7 days post-injury, an effect that was lost in the mitoNEET null animals. (D) Representative images of NL-1 mediated cortical sparing are provided. n=15–16/group, mean ± SEM, One-way ANOVA, Compared Drug Treated to Vehicle within same genotype, Dunnet Post-Hoc, F3,62 = 2.91, * p=0.042.