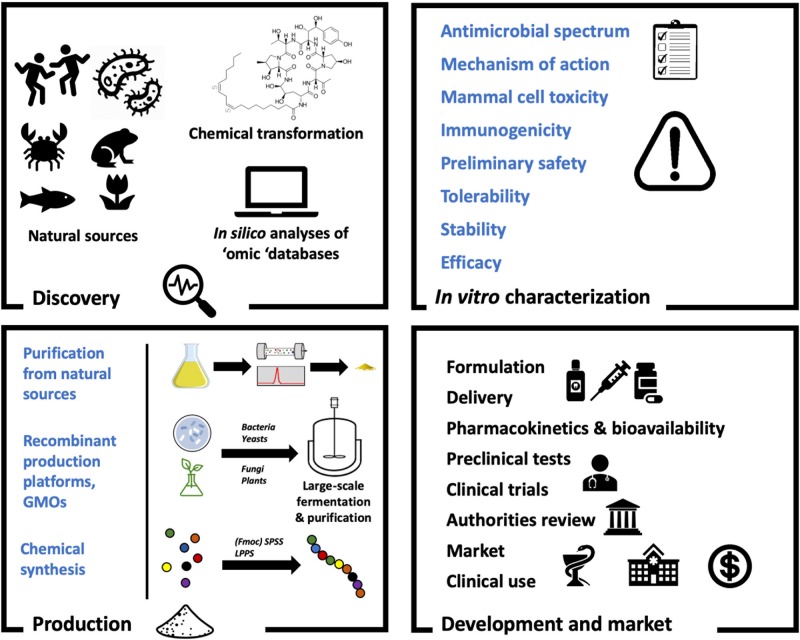
Figure 1.
The antifungal peptide development process. As with any drugs, AFPs must undergo several stages of development to reach clinical use. When the candidate molecule shows promise as a therapeutic (Discovery) it must be characterized (In vitro characterization). In order to facilitate this, sufficient amounts of the peptide must be available (Production). Finally, the molecule will be subjected to formulation processes and preclinical tests before going into clinical trials and receive approval (Development and market).