Abstract
Background
This study aimed to identify the factors that predict distant recurrence and survival outcome after patients with primary positive hormone receptor-positive (HR+) invasive breast cancer undergo complete excision for isolated local recurrence (ILR).
Methods
From January 2000 to December 2009, we performed a retrospective review of our database and identified 51 patients with HR + invasive breast cancer who underwent complete excision for ILR as a component of salvage therapy. The distant metastasis-free survival (DMFS) and overall survival (OS) from the time of ILR were calculated using the Kaplan–Meier method, and a Cox regression model was used for multivariate analysis.
Results
Of the 51 cases of ILR, 28 were of ipsilateral breast tumor recurrence and 23 were of chest wall recurrence. By receiver operating characteristic curve analyses, the cut-off time point for time to ILR was determined to be 29 months. According to time to ILR (≤29 vs. >29 months) and primary tumor size (≤2 vs. >2 cm), patients were divided into four risk groups as variables for analysis. On multivariate analysis, two independent prognostic factors for DMFS and OS after ILR were identified: risk groups (ILR≤29 months with primary tumor size >2 cm vs. ILR>29 months with primary tumor size ≤ 2 cm, HR = 8.53 for DMFS and HR = 11.18 for OS) and primary tumor grade (2/3 vs. 1, HR = 6.10 for DMFS and 4.27 for OS).
Conclusion
We demonstrated that poor DMFS and OS are associated with high risk group defined as short time to ILR (≤29 months) with primary tumor size (>2 cm) and higher primary tumor grade (2/3) among patients with HR + invasive breast cancer treated with complete excision for ILR. Therapeutic strategies for ILR based on hormone therapy with new agents should be explored in future prospective studies, especially for patients with poor outcome.
Keywords: Breast cancer, Excision, Hormone receptor-positive, Isolated local recurrence, Prognosis
At a glance of commentary
Scientific background on the subject
Isolated local recurrence is a heterogeneous cancer and its treatment should be performed with a curative intent. This study aimed to identify the factors that predict distant recurrence and survival outcome after patients with primary positive hormone receptor-positive invasive breast cancer undergo complete excision for isolated local recurrence.
What this study adds to the field
The study demonstrated that short time to ILR (29 months) with larger primary tumor (2 cm) and higher primary tumor grade (2/3) were poor prognostic factors for both distant metastasis-free survival and overall survival in patients with hormone receptor-positive invasive breast cancer treated with complete excision for isolated local recurrence.
Isolated local recurrence (ILR) after breast-conserving surgery (BCS) or mastectomy for invasive breast cancer is considered an adverse prognostic factor associated with disease-free survival (DFS) and overall survival (OS) [1], [2], [3], [4], [5], [6]. The incidence rate of ipsilateral breast tumor recurrence (IBTR) for patients undergoing BCS with radiotherapy and chest wall recurrence (CWR) after mastectomy is 4–10% within 10 years [7], [8], [9], [10]. The reported 5-year survival rates after IBTR and CWR are 45–79% and 24–78%, respectively [7], [8], [9], [10], [11]. Numerous studies have reported that poor DFS and OS are associated with a short interval from diagnosis to ILR (<2 years) [2], [3], [6], [7], [8], [12], [13], more extensive local recurrence [14], [15], and local recurrence with invasive histological components [14] among patients with IBTR. Similarly, for those with CWR, a short interval from diagnosis to ILR (<2 years) [8], [9], [16], [17] and extensive local recurrence [17], [18] are documented to be associated with worse outcomes.
Indeed, ILR is a heterogeneous cancer and may be a source of distant metastases or a sign of systemic spread prior to initial treatment. However, the conventional wisdom that ILR following BCS or mastectomy uniformly confers a dismal prognosis is not necessarily true [18], [19]. At present, most people follow the guidelines for treating ILR patients with a curative intent when possible [20]. Good local control and effective systemic drug therapy are indispensable in developing a treatment plan based on the premise of a curative intent. Regarding local treatment for ILR, to obtain the best local oncological results by wide excision is routinely recommended when possible based on evidence that complete excision of ILR [17], [19], [21] is associated with favorable outcomes. Regarding systemic treatment for ILR, the setting of treatment strategy is usually dependent on the subtypes of primary tumor and recurrent tumor. In case of hormone receptor-negative (HR-) ILR, salvage chemotherapy should be recommended according to the results of the CALOR trial [22]. On the other hand, salvage hormone therapy is preferred in case of hormone receptor-positive (HR+) ILR in view of its expected benefit and low toxicity [20]. In fact, several studies have demonstrated that patients with positive HR status of the primary tumor [9], [17] belong to a subgroup with better outcomes.
Most studies examine prognostic factors and treatment outcomes for local recurrence including different molecular subtypes (HR+ vs. HR–), operability and free safety margins after surgical resection of locally recurrent lesions (complete vs. incomplete). Certainly, patients with HR + breast cancer underwent subsequent complete excision for ILR were considered to be a subgroup with better prognosis in comparison with other subgroups. There is currently no specific study for this subgroup with homogeneity and a relatively good prognosis.
The aim of this study was to identify factors that predict distant metastasis-free survival (DMFS) and OS in patients with primary HR + breast cancer who develop ILR followed by complete excision as a component of salvage treatment.
Materials and methods
Study population
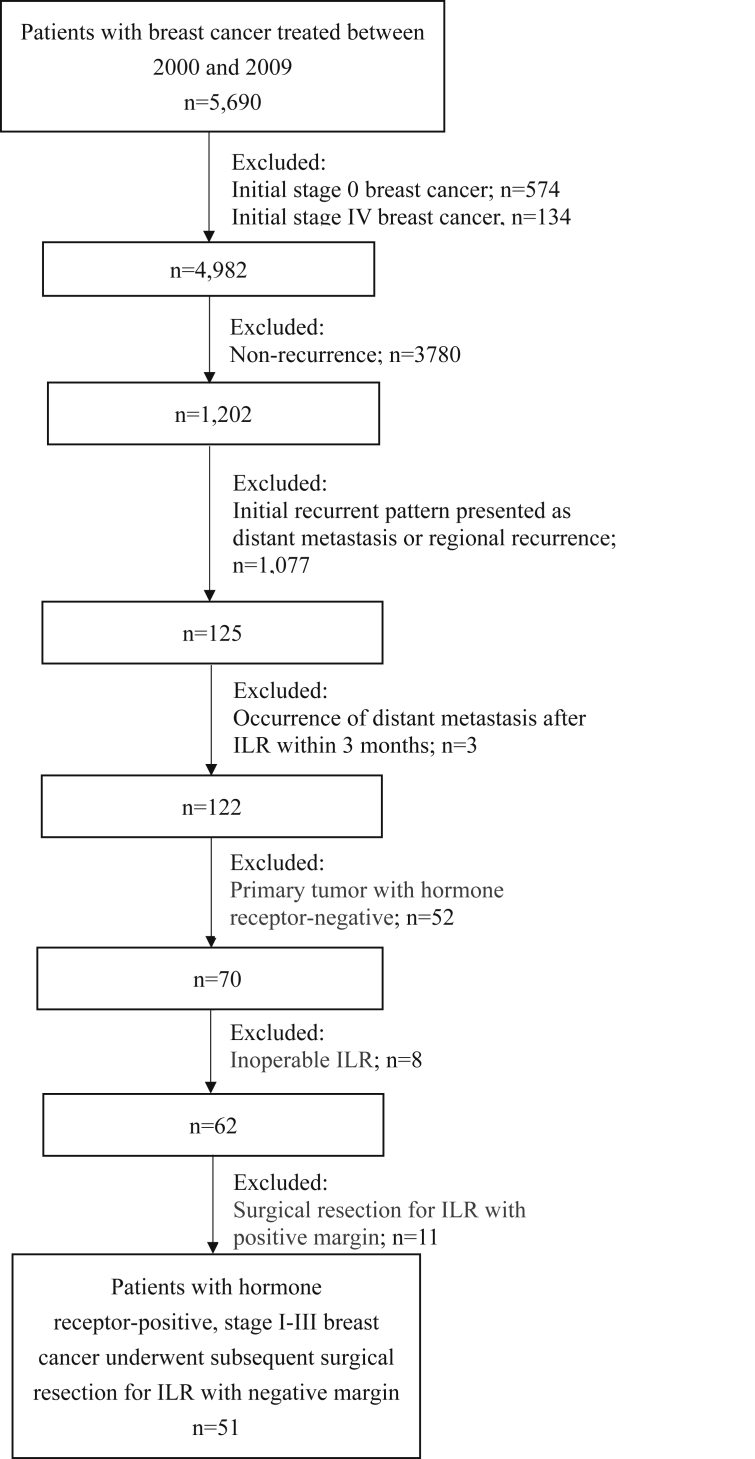
The study was approved by the institutional review board of the relevant institution. There were 5690 women with invasive breast cancer treated between January 1, 2000 and December 31, 2009 identified in the breast cancer database at Chang Gung Memorial Hospital [Fig. 1]. In general, patients were scheduled for regular visits every 3–6 months in the first 2 years, every 6 months in the next three to five years and annually thereafter during follow-up period. Detailed medical history inquiry including symptoms and physical examination were performed at the planned visits. Blood cell counts, routine chemistry tests, and tumor markers such as carcinoembryonic antigen and cancer antigen 15-3 were performed every 6 months in the first five years and annually thereafter. Annual mammography with ultrasound were performed and whenever considered indicated. Once local recurrence was detected, a systemic workup, including computed tomography of the chest, abdomen, and pelvis; and bone scan to detect the presence of a distant metastasis was performed. We conducted a retrospective search of cases from electronic medical records and pathology reports in the database, and identified 122 patients with stage I, II or III invasive breast cancer with a subsequent ILR after BCS or mastectomy. Regarding surgical treatment for the primary tumor, 39.3% (48/122) of patients underwent BCS and 60.70% (74/122) underwent mastectomy. Among the 48 patients who underwent BCS, 39 (81.3%) received postoperative radiotherapy and 9 (18.7%) did not. Of the 74 patients who underwent mastectomy, 24 (32.4%) received postoperative radiotherapy and 50 (67.6%) did not. ILR was defined in this study as a pathologically confirmed recurrence of breast cancer that occurred in the operated breast parenchyma and/or skin or in the skin or muscle of the chest wall, without concurrent accompanying nodal metastasis and/or distant metastasis, and at least three months prior to regional or distant metastasis.
Fig. 1.
Flowchart showing patients excluded from the study. Abbreviation used: ILR: isolated local recurrence.
Histopathological evaluation of the primary and recurrent tumors was performed according to standard procedures. The estrogen receptor (ER) and progesterone receptor (PR) status were determined using immuohistochemical (IHC) with Allred scores, with positive scores ranging from 2 to 8. Human epidermal growth factor receptor 2 (HER2)-positive tumors were defined as IHC3+ or IHC2+ and fluorescent in situ hybridization-positive. Regarding the HR status of primary tumors among the 122 patients who experienced ILR, 46 (37.7%) were ER+ and PR+; 24 (19.7%) were ER– and PR + or ER+ and PR–; and 52 (42.6%) were ER– and PR–. Among the 70 patients with ILR with primary HR + tumors, 62 (88.6%) underwent surgery as a component of salvage treatment and 8 (11.4%) did not. A complete excision was defined here as more than 1 mm negative margin on margin status of the excised specimen after salvage surgical treatment. Therefore, of the 62 patients who underwent subsequent surgery, 51 (82.3%) were identified as having undergone complete excision, and these constituted the current study subjects; the remaining 11 (17.7%) were considered to have undergone incomplete excision and were excluded from the study. Follow-up after complete excision of ILR was carried out as for primary breast cancer. Clinical and histopathological data for these 51 patients, including patient age at diagnosis, initial surgery type, histology of primary and recurrent tumor, primary and recurrent tumor pathological size, primary tumor grade, lymph node (LN) status, staging information, IHC staining of primary tumor, lymphovascular invasion (LVI) of primary tumor, time to local recurrence, all treatment received for the primary tumor and after ILR, dates of disease progression including locoregional recurrence and distant metastasis, and dates of death were retrospectively reviewed.
Statistical analysis
Time to ILR was calculated from initial diagnosis until the occurrence of ILR. Time to DMFS was calculated from salvage surgery until the occurrence of distant metastasis. OS was calculated from the date of salvage surgery until death from breast cancer or the last follow-up. The actuarial DMFS and OS analysis were calculated using the Kaplan–Meier method, and statistical significance was evaluated using the log-rank test. The cut-off time point for time to ILR that is able to discriminate the survival outcome was assessed for the study objects by receiver operating characteristic (ROC) curve, in which the sensitivity (SE) is plotted as a function of 1-specificity (1-SP). The Youden Index (J), one of the main summary statistics of the ROC curve, defines the maximum potential effectiveness of a time point. The cut-off value that achieves this maximum is referred to as the optimal cut-off point that optimizes the discriminating power of the time point when the sensitivity and specificity bear equal weight [23], [24].
The following potential prognostic factors were considered: age at initial diagnosis, time to ILR, histologic characteristics and surgery type of the primary tumor, and histologic characteristics and management of the recurrent tumor. Potentially confounding variables with a p ≤ 0.05 on univariate analysis were incorporated into a Cox proportional hazards model for multivariate analysis. A p value ≤ 0.05 was considered statistically significant. All statistical analyses were performed using SPSS software, version 20 (SPSS Inc., Chicago, IL, USA).
Results
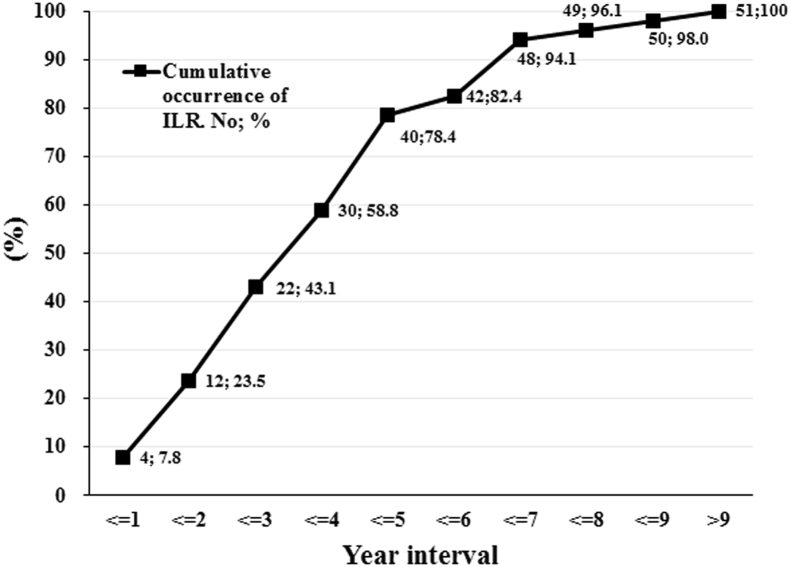
Patient characteristics at initial diagnosis of breast cancer are summarized in Table S1 (see the Supplementary Appendix). The primary tumor histology of these 51 patients consisted of 46 invasive ductal carcinoma (IDC), 3 invasive lobular carcinoma (ILC), 1 tubular carcinoma, and 1 mucinous carcinoma. The cumulative incidence of ILR after initial diagnosis of breast cancer in these 51 patients is shown in [Fig. 2]. The median time from initial diagnosis to ILR was 41.6 months (range, 7.5–150.8 months) and the median follow-up period from the time of ILR was 61.4 months (range, 14.4–130.8 months). Patient characteristics at ILR are summarized in Table S2 (see the Supplementary Appendix). The histology of the recurrent tumors was as follows: 6 ductal carcinoma in situ (DCIS), 40 IDC, 2 ILC, and 3 mucinous carcinoma. We compared the histology of primary and recurrent tumors and observed changes in the histological types of some of them. In 46 cases of primary tumor histology with IDC, the recurrent tumors histological types were 38 IDC, 6 DCIS and 2 mucinous carcinoma. In 3 cases of primary tumor histology with ILC, the recurrent tumors histological types were 1 IDC and 2 ILC. A case of primary tumor histology with mucinous carcinoma was consistent with its recurrent tumor histology. A case of primary tumor histology with tubular carcinoma developed recurrent tumor histology with IDC later. The histological consistency between all primary and recurrent tumors is 80.4% (41/51). Regarding the initial surgical treatment for the primary tumors of these 51 patients, 55% (28/51) patients underwent BCS and 45% (23/51) underwent mastectomy. Among the 28 patients who underwent BCS, 23 (82.1%) received postoperative radiotherapy and 5 (17.9%) did not. Of the 23 patients who underwent mastectomy, 6 (26.1%) received postoperative radiotherapy and 17 (73.9%) did not. In terms of systemic adjuvant therapy for primary cancer, chemotherapy alone was administered in 7 patients, hormone therapy alone was administered in 5 patients, both were administered in 34 patients, and 5 patients received none. Regarding systemic salvage therapy for recurrent disease: 10 patients received chemotherapy alone, 17 received hormone therapy alone, 13 received both, and 11 received none. Ten patients who were treated with chemotherapy alone as the systemic salvage therapy were having been treated with adjuvant hormone therapy after primary tumor surgery. Nineteen of 51 patients received radiotherapy after complete excision of ILR. The recurrence side were IBTR in 4 and CWR in 15.
Fig. 2.
Cumulative incidence of ILR after initial diagnosis of breast cancer in 51 patients. Abbreviation used: ILR: isolated local recurrence.
Pattern of disease progression
Three patients had a second isolated CWR and experienced repeat salvage surgery; all of these were disease-free until the last follow-up. Isolated ipsilateral neck LN metastasis initially occurred in 3 patients and was followed later by distant metastasis. Distant metastasis developed in 24 patients. The sites of distant metastasis were as follows: brain in 1 patient, bone in 9 patients, liver in 5 patients, lung in 12 patients, and nonregional LN(s) in 3 patients.
Disease-free survival and prognostic factors
The 5-year DMFS after ILR was 52.9%. The mean DMFS time after ILR was 79.5 months. We used Younden Index to determine the cut-off of recurrence time to be 29 months. The time to ILR was divided into early and late relapse according to the cut-off time point. Among the clinical and pathologic characteristics from initial diagnosis to ILR, only size of primary tumor (cm) was significantly correlated with time to ILR (p = 0.007, 0dds ratio = 5.76) for early relapse/late relapse, by multiple logistic regression analysis (Table S3 in the Supplementary Appendix).
Since the primary tumor size was the only significant factor for time to ILR and there was a high correlation between the two clinicopathological factors, patients were divided into four risk groups according to time to ILR (≤29 vs. >29 months) and primary tumor size (≤2 vs. >2 cm). Patients with smaller primary tumors (≤2 cm) who experienced short time to ILR (≤29 months) are classified as group A; patients with smaller primary tumors (≤2 cm) who experienced long time to ILR (>29 months) are classified as group B; patients with larger primary tumors (>2 cm) who experienced short time to ILR (≤29 months) are classified as group C; and patients with larger primary tumors (>2 cm) who experienced long time to ILR (>29 months) are classified as group D.
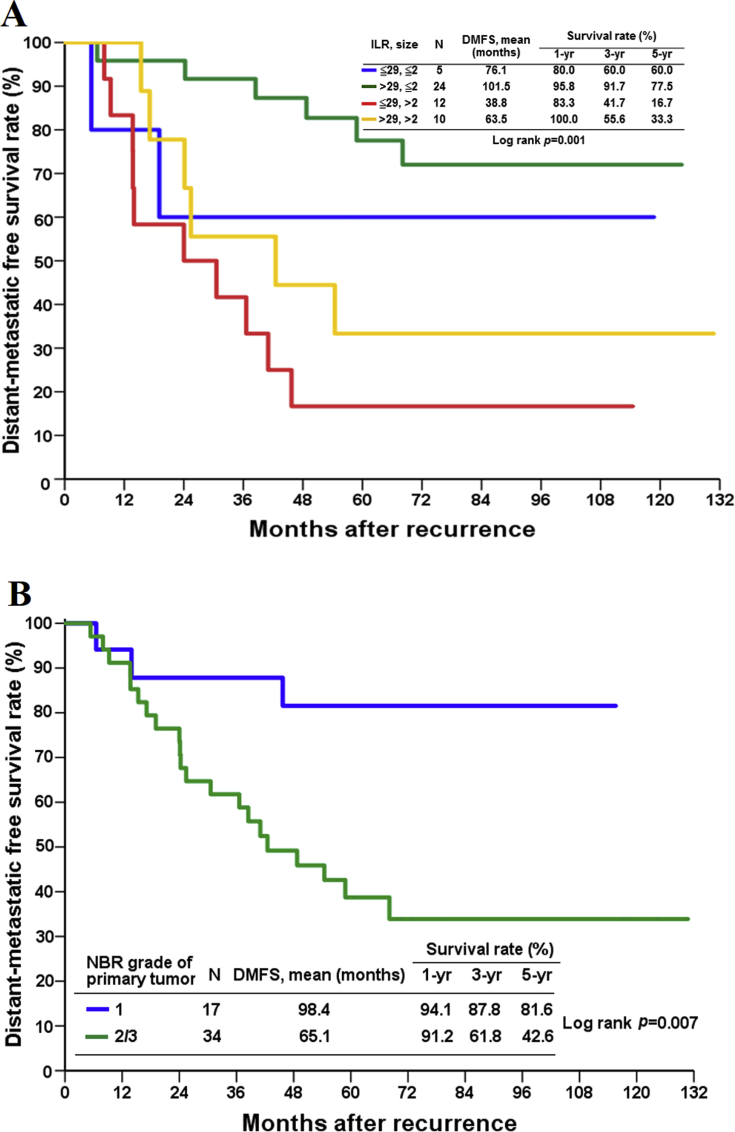
We conducted a DMFS analysis to identify factors associated with treatment failure after ILR. In univariate analysis, time to ILR (≤29 vs. >29 months) (p = 0.004), primary tumor size (≤2 vs. >2 cm) (p < 0.001), risk groups (p = 0.001), and primary tumor grade (1 vs. 2/3) (p = 0.007) had a significant influence on DMFS, whereas LN status of primary tumor (negative vs. positive) (p = 0.381), recurrent tumor size (≤1 vs.>1 cm) (p = 0.216) and salvage treatment with the exception of surgery after ILR, including chemotherapy (p = 0.130), hormone therapy (p = 0.645), and radiotherapy (p = 0.083) did not [Table 1 & Fig. 3]. On multivariate analysis, two independent prognostic factors for DMFS after ILR were identified: risk groups (group C vs. group B, hazard ratio: 8.53) and primary tumor grade (2/3 vs. 1, hazard ratio: 6.10) [Table 1]. Group C patients had a mean DMFS of 38.8 months, which was significantly shorter than the DMFS of group B patients (101.5 months) ([Fig. 3A], p = 0.001, log-rank test). As for the mean DMFS of group A and group D, although longer than group B, it is not statistically significant.
Table 1.
Univariate and multivariate analysis of factors influencing the DMFS in breast cancer patients with ILR.
| Factors | DMFS (%) |
p value | Multivariate analysisa |
p value | |||
|---|---|---|---|---|---|---|---|
| 3-yr | 5-yr | HR | 95% CI of HR | ||||
| Age at initial | ≦40 (n = 13) | 84.6 | 67.7 | 0.406 | – | ||
| Diagnosis (years) | >40 (n = 38) | 65.0 | 48.0 | ||||
| Time interval from initial diagnosis to ILR (months) | ≦29 (n = 17) | 47.1 | 29.4 | 0.004 | – | ||
| >29 (n = 34) | 81.9 | 65.1 | |||||
| Size of primary tumor (cm) | ≦2 (n = 29) | 86.2 | 74.5 | <0.001 | – | ||
| >2 (n = 22) | 47.8 | 23.9 | |||||
| Risk groupsb | Group A (≦29, ≦2) (n = 5) | 60.0 | 60.0 | 0.001 | 1.77 | 0.36–8.81 | 0.484 |
| Group B (>29, ≦2) (n = 24) | 91.7 | 77.5 | 1 | ||||
| Group C (≦29, >2) (n = 12) | 41.7 | 16.7 | 8.53 | 2.87–25.34 | <0.001 | ||
| Group D (>29, >2) (n = 10) | 55.6 | 33.3 | 2.30 | 0.73–7.25 | 0.156 | ||
| Initial surgery type | Mastectomy (n = 23) | 60.9 | 43.5 | 0.144 | – | ||
| BCS (n = 28) | 78.0 | 61.8 | |||||
| NBR grade of primary tumor | 1 (n = 17) | 87.8 | 81.6 | 0.007 | 1 | ||
| 2/3 (n = 34) | 61.8 | 42.6 | 6.10 | 1.67–22.23 | 0.006 | ||
| ER status of primary tumor | Negative (n = 3) | 66.7 | 33.3 | 0.709 | – | ||
| Positive (n = 48) | 70.3 | 54.5 | |||||
| PR status of primary tumor | Negative (n = 15) | 50.9 | 36.4 | 0.129 | – | ||
| Positive (n = 36) | 77.8 | 59.8 | |||||
| Her-2 status of primary tumor | Negative (n = 37) | 69.6 | 57.8 | 0.598 | – | ||
| Positive (n = 14) | 71.4 | 40.0 | |||||
| LN status of primary tumor | Negative (n = 22) | 81.1 | 56.1 | 0.381 | – | ||
| Positive (29) | 62.1 | 50.4 | |||||
| Recurrent tumor size (cm) | ≦1 (n = 25) | 72.0 | 63.6 | 0.216 | – | ||
| >1 (n = 26) | 68.2 | 43.8 | |||||
| Chemotherapy after ILR | No (n = 28) | 55.8 | 43.3 | 0.130 | – | ||
| Yes (n = 23) | 87.0 | 63.9 | |||||
| Hormone therapy after ILR | No (n = 21) | 76.2 | 53.2 | 0.645 | – | ||
| Yes (n = 30) | 65.7 | 51.9 | |||||
| Radiotherapy after ILR | No (n = 32) | 77.6 | 60.8 | 0.083 | – | ||
| Yes (n = 19) | 57.9 | 40.5 | |||||
Abbreviations: DMFS: distant-metastatic free survival; ILR: isolated local recurrence; BCS: breast-conserving surgery; NBR: Nottingham Bloom Richardson; ER: estrogen receptor; PR: progesterone receptor; Her-2: human epidermal growth factor receptor 2; LN: lymph node; CI: confidence interval.
Multivariate analysis using Cox proportional hazard model.
Risk group classification according to time to ILR (≦29 vs. >29 months) and primary tumor size (≦2 vs. >2 cm).
Fig. 3.
Estimated cumulative incidence of distant-metastatic free survival according to (A) risk group classification according to time to ILR (≤29 vs. >29 months) and primary tumor size (≤2 vs. >2 cm) (B) NBR grade of primary tumor. Abbreviations used: DMFS: distant-metastatic free survival; NBR: Nottingham Bloom Richardson; ILR: isolated local recurrence.
Overall survival and prognostic factors
The mean survival time after ILR was 91.9 months. The 5-year OS after ILR was 68%. The influence on survival of 14 variables was evaluated using univariate analysis. Time to ILR (≤29 vs. >29 months) (p = 0.001), primary tumor size (≤2 vs. >2 cm) (p < 0.001), risk groups (p < 0.001), and initial tumor grade (1 vs. 2/3) (p = 0.019) were significant prognostic factors affecting survival, whereas LN status of primary tumor (negative vs. positive) (p = 0.137), recurrent tumor size (≤1 vs.>1 cm) (p = 0.548) and other treatments with the exception of surgery after ILR, including chemotherapy (p = 0.073), hormone therapy (p = 0.706), and radiotherapy (p = 0.108) were not [Table 2].
Table 2.
Univariate and multivariate analysis of factors influencing the OS in breast cancer patients with ILR.
| Factors | OS (%) |
p value | Multivariate analysisa |
p value | |||
|---|---|---|---|---|---|---|---|
| 3-yr | 5-yr | HR | 95% CI of HR | ||||
| Age at initial Diagnosis (years) |
≦40 (n = 13) | 92.3 | 83.9 | 0.240 | – | ||
| >40 (n = 38) | 76.3 | 62.5 | |||||
| Time interval from initial diagnosis to ILR (months) | ≦29 (n = 17) | 64.7 | 40.3 | 0.001 | – | ||
| >29 (n = 34) | 88.2 | 81.5 | |||||
| Size of primary tumor (cm) | ≦2 (n = 29) | 93.1 | 85.7 | <0.001 | – | ||
| >2 (n = 22) | 63.6 | 50.0 | |||||
| Risk groupsb | Group A (≦29, ≦2) (n = 5) | 80.0 | 60.0 | <0.001 | 3.05 | 0.51–18.39 | 0.224 |
| Group B (>29, ≦2) (n = 24) | 95.8 | 85.9 | 1 | ||||
| Group C (≦29, >2) (n = 12) | 58.3 | 33.3 | 11.18 | 3.04–41.12 | <0.001 | ||
| Group D (>29, >2) (n = 10) | 70.0 | 70.0 | 3.39 | 0.80–14.42 | 0.098 | ||
| Initial surgery type | Mastectomy (n = 23) | 73.9 | 60.3 | 0.265 | – | ||
| BCS (n = 28) | 85.7 | 73.3 | |||||
| NBR grade of primary tumor | 1 (n = 17) | 94.1 | 81.9 | 0.019 | 1 | ||
| 2/3 (n = 34) | 73.5 | 60.0 | 4.27 | 1.20–15.29 | 0.026 | ||
| ER status of primary tumor | Negative (n = 3) | 66.7 | 66.7 | 0.817 | – | ||
| Positive (n = 48) | 81.2 | 67.4 | |||||
| PR status of primary tumor | Negative (n = 15) | 73.3 | 52.5 | 0.145 | – | ||
| Positive (n = 36) | 83.3 | 74.0 | |||||
| Her-2 status of primary tumor | Negative (n = 37) | 78.4 | 69.5 | 0.812 | – | ||
| Positive (n = 14) | 85.7 | 61.2 | |||||
| LN status of primary tumor | Negative (n = 22) | 90.9 | 76.4 | 0.137 | – | ||
| Positive (29) | 72.4 | 60.7 | |||||
| Recurrent tumor size (cm) | ≦1 (n = 25) | 80.0 | 71.1 | 0.548 | – | ||
| >1 (n = 26) | 80.8 | 65.0 | |||||
| Chemotherapy after ILR | No (n = 28) | 75.0 | 60.0 | 0.073 | – | ||
| Yes (n = 23) | 87.0 | 76.7 | |||||
| Hormone therapy after ILR | No (n = 21) | 85.7 | 68.2 | 0.706 | – | ||
| Yes (n = 30) | 76.7 | 66.5 | |||||
| Radiotherapy after ILR | No (n = 32) | 87.5 | 73.3 | 0.108 | – | ||
| Yes (n = 19) | 68.4 | 57.0 | |||||
Abbreviations: OS: overall survival; ILR: isolated local recurrence; BCS: breast-conserving surgery; NBR: Nottingham Bloom Richardson; ER: estrogen receptor; PR: progesterone receptor; Her-2: human epidermal growth factor receptor 2; LN: lymph node; CI: confidence interval.
Multivariate analysis using Cox proportional hazard model.
Risk group classification according to time to ILR (≦29 vs. >29 months) and primary tumor size (≦2 vs. >2 cm).
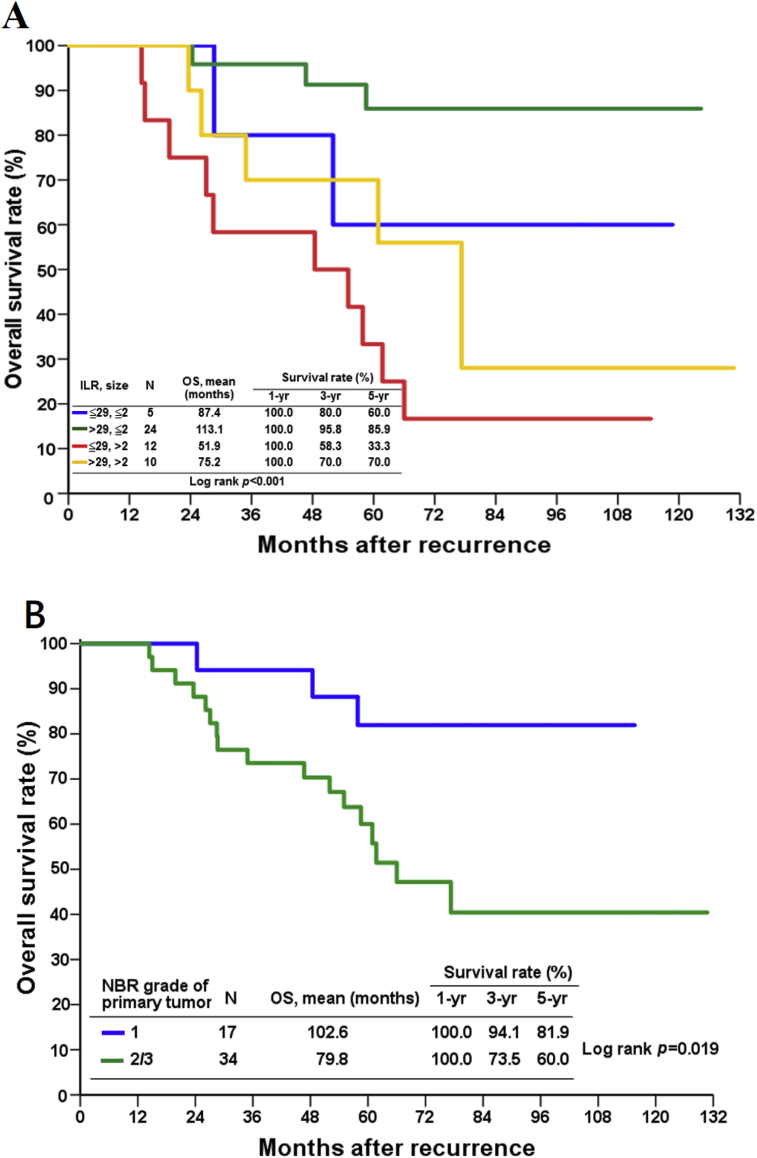
On multivariate analysis, two independent prognostic factor were found to have significant effects on survival outcome: risk groups (group C vs. group B, hazard ratio: 11.18) and primary tumor grade (2/3 vs. 1, hazard ratio: 4.27) [Table 2 and Fig. 4]. Group C patients had a mean survival of 51.9 months, which was significantly shorter than the survival of group B patients (113.1 months) ([Fig. 4A], p < 0.001, log-rank test). Although the mean survival of group A and group D patients is longer than that of the group B patients, it is not statistically significant.
Fig. 4.
Estimated cumulative incidence of overall survival according to (A) risk group classification according to time to ILR (≤29 vs. >29 months) and primary tumor size (≤2 vs. >2 cm) (B) NBR grade of primary tumor. Abbreviations used: OS: overall survival; NBR: Nottingham Bloom Richardson; ILR: isolated local recurrence.
Discussion
In the present study, we evaluated the DMFS and OS among 51 patients with primary HR + tumor who underwent complete excision of ILR and identified that primary tumor grade and risk groups stratification based on primary tumor size and time to ILR are independent factors for both DMFS and OS. We determined that the cut-off time point for time to ILR in HR + breast cancer patients is different from the commonly used interval of twenty-four months and longer. To the best of our knowledge, there have been no previous studies focused on patients with such favorable outcomes to date. Moreover, the identified prognostic factors in the current study might be useful for stratification in developing treatment strategies.
In the current study, the 5-year DMFS and OS after salvage surgery were 52.9% and 68%, which are comparable to previously published findings [6], [25]. Wapnir et al. reported that the 5-year DMFS and OS rates in patients who experienced local recurrence were 51% and 60%, respectively [6]. Similarly, Montagna et al. observed that the 5-year DFS and OS rates in patients with local recurrence were 45% and 71%, respectively [25]. From our findings, it was known that among patients with HR + breast cancer experienced ILR, even if the local recurrence lesion was completely removed to achieve local control, nearly half of the patients would have a distant metastasis within five years. This suggests that identifying high-risk factors for this group of patients and planning effective treatment strategies for these patients with an unpredictable prognosis.
Several clinicopathological factors associated with DMFS and OS for patients with local recurrence have been addressed in numerous studies.
Time to ILR is the most frequently reported prognostic factor for DMFS and OS in patients with breast cancer with ILR [2], [3], [6], [7], [8], [9], [12], [13], [16], [17], [26]. Although different time intervals have been used in the past literatures, such as one year, two years or even five years, the length of time to ILR at two-year intervals is still the most commonly used [3], [6], [9], [12], [13], [16], [17]. However, researchers in various studies have found that the time point of recurrence is related to the HR status, in HR-breast cancer patients, most recurrences during the first 3 years of follow up, whereas in HR + breast cancer patients, a significant number of recurrences occur in subsequent years after 5 years of hormone therapy [27]. Actually, length of time to ILR reflects not only the nature of the tumor itself, the aggressiveness and characteristics, but also the effectiveness of the hormone therapy. Taking into account the HR status to determine the clinically relevant threshold of time to ILR is necessary. Gosset et al. reported a study involving 2209 patients with breast invasive carcinoma who were treated with BCS followed for local recurrence [26]. The authors found that different prognostic times to ILR for DMFS between HR+ and HR-breast cancer patients: 49 months in the HR + group and 33 months in the HR-group. In the present study, we identified 29 months as the cut-off for time to ILR that best discriminate the DMFS and OS outcome. Of note, patients who were diagnosed with distant metastases within 3 months of ILR which were usually considered as synchronous metastases were included the study reported by Gosset et al. but excluded in our study. Such different results explained the different time point of time to ILR depending on different study objects. Owing to the small numbers of patients in our series, the optimal cut-off time point for time to ILR for HR + breast cancer patients is may be revised after inclusion of more cases.
It can be reasonably inferred that there is a correlation between the time interval between local recurrence and the follow-up sequence. Although there is still no randomized data to support any particular follow-up sequence, we followed the recommendations of European Society for Medical Oncology (ESMO) clinical practice guidelines for primary breast cancer follow-up [28]. In our series, half of the patients were found to have small tumor size (≤1 cm) at local recurrence. The result might be related to the regular follow-up sequence we used. In addition, the size of the primary tumor was the only factor related to the time interval between local recurrences in our series. Therefore, when scheduling follow-up sequence, especially after surgery of larger primary tumors, regular local imaging modalities such as mammography and breast ultrasound should be considered.
Primary tumor size and primary tumor grading have been shown in several studies as significant prognostic factors for patients with ILR, similar to what we report here [6], [26], [29]. Gosset et al. reported that the primary tumor size and grade for HR + tumors were significantly correlated with the DMFS, which were consistent with our findings. In addition, we observed a high correlation between the primary tumor size and time to ILR. Further stratification of these two factors, successfully identified that patients with large primary tumors in an experienced short time to ILR was the risk group with the worst prognosis compared with other groups. In the present study, risk groups and tumor grade were significantly associated with DMFS and OS prognosis, both of which could be used as a stratification for risk classification.
Since the treatment failure pattern is mainly presented by distant metastasis, how to develop more effective systemic treatment is quite important. Regarding systemic treatment, the CALOR trial reported that the administration of salvage chemotherapy after an isolated locoregional recurrence was significantly more effective for women with ER-disease. As for ER + disease, the benefit of salvage chemotherapy in combination with hormone therapy remained uncertain in their cohort [22]. In our cohort, the use of salvage chemotherapy was not found to shown any benefit on DMFS and OS. Waeber et al. reported a trial involving 167 patients who underwent radical surgery and radiotherapy for locoregional recurrence among those treated with mastectomy initially. The authors compared tamoxifen therapy with observation and showed that tamoxifen therapy had a beneficial effect on DFS but not on OS [30]. There remains a lack of published reports to demonstrate that the administration of salvage hormone therapy results in a statistically significant improvement in subsequent outcomes among patients with IBTR. In the present study, salvage hormone therapy administered after complete excision of ILR was not found to improve DMFS and OS, which is consistent with the findings of several reported studies [6], [17], [31]. Despite this, salvage hormone therapy in patients with HR+ is justified owing to its expected benefit and low toxicity [20]. Current adjuvant hormone therapy includes selective estrogen-receptor modulators (SERMs), aromatase inhibitors (AIs), ovarian suppression and a selective estrogen-receptor degrader (fulvestrant). In our series, each patient received at least one or more hormone therapy drugs including tamoxifen or AIs before or after local recurrence but none of them received ovarian suppression or fulvestrant treatment. The updated results from the joint analysis of Suppression of Ovarian Function Trial (SOFT) and the Tamoxifen and Exemestane Trial (TEXT) found that among premenopausal women with ER + breast cancer, the combination of ovarian suppression and tamoxifen significantly reduced recurrence, compared with tamoxifen alone [32]. Further improvement was seen with the combination of ovarian suppression and exemestane. For patients with Her2-negative breast cancer with high-risk features can derive a meaningful improvement in 8-year DFS (particularly distant metastasis) with exemestane plus ovarian suppression, as an alternative to tamoxifen. Such results may be applied to salvage hormone therapy in premenopausal patients, especially those at high risk. For menopausal patients who had not received previous hormone therapy, fulvestrant may be an alternative to hormone therapy. The FALCON study compared fulvestrant with anastrozole as the first hormone therapy in patients with HR + locally advanced or metastatic breast cancer who had not received previous hormone therapy and demonstrated the greater efficacy of fulvestrant over anastrozole in the hormone therapy naïve setting [33]. For menopausal patients who had received previous hormone therapy, several combination therapies can be considered. The combination of AIs with a CDK4/6 inhibitor as a treatment option for first line therapy of advanced ER + Her2-breast cancer in postmenopausal patients is indicated by the positive results from MONALEESA-2 and PALOMA-2 [34], [35]. Other options include the combination of palbociclib with fulvestrant (PALOMA-3 regimen) and the combination of exemestane with everolimus (BOLERO-2 regimen) [36], [37].
However, the incorporation of these new agent in the treatment of patients with HR + breast cancer with local recurrence are still needed prospective studies to carefully evaluate the benefits and risks of them.
The current study had several limitations. First, because it was a retrospective analysis performed at a single institution, treatment and patient characteristics were heterogeneous. Selection bias may exist for the clinical physicians concerning the decision of salvage treatment for individual patients. Owing to a variety of salvage chemotherapy and hormone therapy regimen drugs were used, made analysis of results limited. Second, because only small proportion of the patients (4 of 14, 28.6%) with HER2-positive breast cancer received anti HER2 therapy as salvage treatment for ILR, we could not analyze the impact of anti HER2 therapy on DMFS and OS outcome in our study. By today's standards adjuvant systemic therapy would have been given to a greater portion of the patients. However, despite the progress in breast cancer treatment over the years since these patients first presented, the message is clear that even in what we consider the patients in good prognosis, early local recurrence has to be kept as a warning sign of a more aggressive systemic disease.
Conclusion
Our study suggests that the cut-off time point for time to ILR in HR + breast cancer patients is 29 months, that is longer than the commonly used interval of 24 months. Larger primary tumors (>2 cm) have a higher likelihood of short time to ILR. The current study demonstrated that larger primary tumor (>2 cm) recurrence in a short time (≤29 months) and higher primary tumor grade (2/3) were poor prognostic factor for both DMFS and OS in patients with HR + invasive breast cancer treated with complete excision for ILR. Hormone therapy is the first choice of treatment for ILR according to past mediations and menstrual status. Newly effective agents should be explored in future prospective studies, especially for patients with poor outcome.
Funding
This study was supported by grants from Chang Gung Hospital Research Program (CORPG1F0051).
Ethics approval and consent to participate
The study was approved by the Chang Gung Foundation Institutional Review Board (IRB No: 201601333B0).
Conflicts of interest
All authors declare they have no conflicts of interest.
Footnotes
Peer review under responsibility of Chang Gung University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bj.2019.07.002.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Fisher B., Anderson S., Fisher E., Redmond C., Wickerham D., Wolmark N. Significance of ipsilateral breast tumour recurrence after lumpectomy. Lancet. 1991;338:327–331. doi: 10.1016/0140-6736(91)90475-5. [DOI] [PubMed] [Google Scholar]
- 2.Whelan T., Clark R., Roberts R., Levine M., Foster G. Ipsilateral breast tumor recurrence postlumpectomy is predictive of subsequent mortality: results from a randomized trial. Int J Radiat Oncol Biol Phys. 1994;30:11–16. doi: 10.1016/0360-3016(94)90513-4. [DOI] [PubMed] [Google Scholar]
- 3.Veronesi U., Marubini E., Vecchio M.D., Manzari A., Andreola S., Greco M. Local recurrences and distant metastases after conservative breast cancer treatments: partly independent events. JNCI J Natl Cancer Inst. 1995;87:19–27. doi: 10.1093/jnci/87.1.19. [DOI] [PubMed] [Google Scholar]
- 4.Fodor J., Polgar C., Major T., Mangel L., Szakolczai I., Szamel I. The time-course of metastases from breast cancer after mastectomy and breast-conserving surgery with and without isolated local–regional recurrence. Breast. 2002;11:53–57. doi: 10.1054/brst.2001.0362. [DOI] [PubMed] [Google Scholar]
- 5.Buchanan C.L., Dorn P.L., Fey J., Giron G., Naik A., Mendez J. Locoregional recurrence after mastectomy: incidence and outcomes. J Am Coll Surg. 2006;203:469–474. doi: 10.1016/j.jamcollsurg.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 6.Wapnir I.L., Anderson S.J., Mamounas E.P., Geyer C.E., Jr., Jeong J.H., Tan-Chiu E. Prognosis after ipsilateral breast tumor recurrence and locoregional recurrences in five National Surgical Adjuvant Breast and Bowel Project node-positive adjuvant breast cancer trials. J Clin Oncol. 2006;24:2028–2037. doi: 10.1200/JCO.2005.04.3273. [DOI] [PubMed] [Google Scholar]
- 7.Haffty B.G., Fischer D., Beinfield M., McKhann C. Prognosis following local recurrence in the conservatively treated breast cancer patient. Int J Radiat Oncol Biol Phys. 1991;21:293–298. doi: 10.1016/0360-3016(91)90774-x. [DOI] [PubMed] [Google Scholar]
- 8.van Dongen J.A., Voogd A.C., Fentiman I.S., Legrand C., Sylvester R.J., Tong D. Long-term results of a randomized trial comparing breast-conserving therapy with mastectomy: European Organization for Research and Treatment of Cancer 10801 trial. JNCI J Natl Cancer Inst. 2000;92:1143–1150. doi: 10.1093/jnci/92.14.1143. [DOI] [PubMed] [Google Scholar]
- 9.Schmoor C., Sauerbrei W., Bastert G., Schumacher M., Group G.B.C.S. Role of isolated locoregional recurrence of breast cancer: results of four prospective studies. J Clin Oncol. 2000;18:1696–1708. doi: 10.1200/JCO.2000.18.8.1696. [DOI] [PubMed] [Google Scholar]
- 10.Arriagada R., Lê M.G., Rochard F., Contesso G. Conservative treatment versus mastectomy in early breast cancer: patterns of failure with 15 years of follow-up data. Institut Gustave-Roussy Breast Cancer Group. J Clin Oncol. 1996;14:1558–1564. doi: 10.1200/JCO.1996.14.5.1558. [DOI] [PubMed] [Google Scholar]
- 11.Grischke E.M., Wallwiener D., Souchon R., Fehm T., Loehberg C., Jud S. Isolated loco-regional recurrence of breast cancer–established and innovative therapy concepts. Geburtsh Frauenheilk. 2013;73:611–622. [Google Scholar]
- 12.Fredriksson I., Liljegren G., Arnesson L.-G., Emdin S., Palm-Sjövall M., Fornander T. Local recurrence in the breast after conservative surgery—a study of prognosis and prognostic factors in 391 women. Eur J Cancer. 2002;38:1860–1870. doi: 10.1016/s0959-8049(02)00219-8. [DOI] [PubMed] [Google Scholar]
- 13.Doyle T., Schultz D.J., Peters C., Harris E., Solin L.J. Long-term results of local recurrence after breast conservation treatment for invasive breast cancer. Int J Radiat Oncol Biol Phys. 2001;51:74–80. doi: 10.1016/s0360-3016(01)01625-x. [DOI] [PubMed] [Google Scholar]
- 14.Voogd A.C., van Tienhoven G., Peterse H.L., Crommelin M.A., Rutgers E.J.T., van de Velde C.J. Local recurrence after breast conservation therapy for early stage breast carcinoma. Cancer. 1999;85:437–446. doi: 10.1002/(sici)1097-0142(19990115)85:2<437::aid-cncr23>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 15.Gage I., Schnitt S.J., Recht A., Abner A., Come S., Shulman L.N. Skin recurrences after breast-conserving therapy for early-stage breast cancer. J Clin Oncol. 1998;16:480–486. doi: 10.1200/JCO.1998.16.2.480. [DOI] [PubMed] [Google Scholar]
- 16.Kuo S.H., Huang C.S., Kuo W.H., Cheng A.L., Chang K.J., Cheng J.C.-H. Comprehensive locoregional treatment and systemic therapy for postmastectomy isolated locoregional recurrence. Int J Radiat Oncol Biol Phys. 2008;72:1456–1464. doi: 10.1016/j.ijrobp.2008.03.042. [DOI] [PubMed] [Google Scholar]
- 17.Willner J., Kiricuta I.C., Kölbl O. Locoregional recurrence of breast cancer following mastectomy: always a fatal event? Results of univariate and multivariate analysis. Int J Radiat Oncol Biol Phys. 1997;37:853–863. doi: 10.1016/s0360-3016(96)00556-1. [DOI] [PubMed] [Google Scholar]
- 18.Halverson K.J., Perez C.A., Kuske R.R., Garcia D.M., Simpson J.R., Fineberg B. Survival following locoregional recurrence of breast cancer: univariate and multivariate analysis. Int J Radiat Oncol Biol Phys. 1992;23:285–291. doi: 10.1016/0360-3016(92)90743-2. [DOI] [PubMed] [Google Scholar]
- 19.Fodor J., Major T., Polgár C., Orosz Z., Sulyok Z., Kasler M. Prognosis of patients with local recurrence after mastectomy or conservative surgery for early-stage invasive breast cancer. Breast. 2008;17:302–308. doi: 10.1016/j.breast.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 20.Cardoso F., Harbeck N., Fallowfield L., Kyriakides S., Senkus E. Group EGW. Locally recurrent or metastatic breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23(S 7):vii11–vii19. doi: 10.1093/annonc/mds232. [DOI] [PubMed] [Google Scholar]
- 21.Galper S., Blood E., Gelman R., Abner A., Recht A., Kohli A. Prognosis after local recurrence after conservative surgery and radiation for early-stage breast cancer. Int J Radiat Oncol Biol Phys. 2005;61:348–357. doi: 10.1016/j.ijrobp.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 22.Aebi S., Gelber S., Anderson S.J., Láng I., Robidoux A., Martín M. Chemotherapy for isolated locoregional recurrence of breast cancer (CALOR): a randomised trial. Lancet Oncol. 2014;15:156–163. doi: 10.1016/S1470-2045(13)70589-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Faraggi D. The effect of random measurement error on receiver operating characteristic (ROC) curves. Stat Med. 2000;19:61–70. doi: 10.1002/(sici)1097-0258(20000115)19:1<61::aid-sim297>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 24.Hajian-Tilaki K. Receiver operating characteristic (ROC) curve analysis for medical diagnostic test evaluation. Caspian J Intern Med. 2013;4:627–635. [PMC free article] [PubMed] [Google Scholar]
- 25.Montagna E., Bagnardi V., Rotmensz N., Viale G., Renne G., Cancello G. Breast cancer subtypes and outcome after local and regional relapse. Ann Oncol. 2011;23:324–331. doi: 10.1093/annonc/mdr129. [DOI] [PubMed] [Google Scholar]
- 26.Gosset M., Hamy A.-S., Mallon P., Delomenie M., Delomenie M., Mouttet D. Prognostic impact of time to ipsilateral breast tumor recurrence after breast conserving surgery. PLoS One. 2016;11:e0159888. doi: 10.1371/journal.pone.0159888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ribelles N., Perez-Villa L., Jerez J.M., Pajares B., Vicioso L., Jimenez B. Pattern of recurrence of early breast cancer is different according to intrinsic subtype and proliferation index. Breast Cancer Res. 2013;15:R98. doi: 10.1186/bcr3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Senkus E., Kyriakides S., Ohno S., Penault-Llorca F., Poortmans P., Rutgers E.6. ESMO Guidelines Committee. Primary breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26:v8–v30. [Google Scholar]
- 29.Panet-Raymond V., Truong P.T., Alexander C., Lesperance M., McDonald R.E., Watson P.H. Clinicopathologic factors of the recurrent tumor predict outcome in patients with ipsilateral breast tumor recurrence. Cancer. 2011;117:2035–2043. doi: 10.1002/cncr.25767. [DOI] [PubMed] [Google Scholar]
- 30.Waeber M., Castiglione-Gertsch M., Dietrich D., Thürlimann B., Goldhirsch A., Brunner K. Adjuvant therapy after excision and radiation of isolated postmastectomy locoregional breast cancer recurrence: definitive results of a phase III randomized trial (SAKK 23/82) comparing tamoxifen with observation. Ann Oncol. 2003;14:1215–1221. doi: 10.1093/annonc/mdg347. [DOI] [PubMed] [Google Scholar]
- 31.Chagpar A., Meric-Bernstam F., Hunt K.K., Ross M.I., Cristofanilli M., Singletary S.E. Chest wall recurrence after mastectomy does not always portend a dismal outcome. Ann Surg Oncol. 2003;10:628–634. doi: 10.1245/aso.2003.01.004. [DOI] [PubMed] [Google Scholar]
- 32.Francis P.A., Pagani O., Fleming G.F., Walley B.A., Colleoni M., Láng I. Tailoring adjuvant endocrine therapy for premenopausal breast cancer. N Engl J Med. 2018;379:122–137. doi: 10.1056/NEJMoa1803164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Robertson J.F., Bondarenko I.M., Trishkina E., Dvorkin M., Panasci L., Manikhas A. Fulvestrant 500 mg versus anastrozole 1 mg for hormone receptorpositive advanced breast cancer (FALCON): an international, randomised, double-blind, phase 3 trial. Lancet. 2016;388:2997–3005. doi: 10.1016/S0140-6736(16)32389-3. [DOI] [PubMed] [Google Scholar]
- 34.Hortobagyi G.N., Stemmer S.M., Burris H.A., Yap Y.S., Sonke G.S., Paluch-Shimon S. Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N Engl J Med. 2016;375:1738–1748. doi: 10.1056/NEJMoa1609709. [DOI] [PubMed] [Google Scholar]
- 35.Finn R.S., Martin M., Rugo H.S., Jones S., Im S.A., Gelmon K. Palbociclib and letrozole in advanced breast cancer. N Engl J Med. 2016;375:1925–1936. doi: 10.1056/NEJMoa1607303. [DOI] [PubMed] [Google Scholar]
- 36.Cristofanilli M., Turner N.C., Bondarenko I., Ro J., Im S.A., Masuda N. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2016;17:425–439. doi: 10.1016/S1470-2045(15)00613-0. [DOI] [PubMed] [Google Scholar]
- 37.Baselga J., Campone M., Piccart M., Burris H.A., 3rd, Rugo H.S., Sahmoud T. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med. 2012;366:520–529. doi: 10.1056/NEJMoa1109653. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.