Abstract
Background
Dyspnea is prevalent among hospitalized patients but little is known about the experience of dyspnea among inpatients. We sought to characterize the multiple sensations and associated emotions of dyspnea in patients admitted with dyspnea to a tertiary care hospital.
Methods
We selected patients who reported breathing discomfort of at least 4/10 on admission (10 = unbearable). Research staff recruited 156 patients within 24 hours of admission and evaluated daily patients’ current and worst dyspnea with the Multidimensional Dyspnea Profile; patients participated in the study 2.6 days on average. The Multidimensional Dyspnea Profile assesses overall breathing discomfort (A1), intensity of five sensory qualities of dyspnea, and 5 negative emotional responses to dyspnea. Patients were also asked to rate whether current levels of dyspnea were “acceptable.”
Results
At the time of the first research interview, patients reported slight to moderate dyspnea (A1 median 4); however, most patients reported experiencing severe dyspnea in the 24 hours before the interview (A1 mean 7.8). A total of 54% of patients with dyspnea ≥4 on day 1 found the symptom unacceptable. The worst dyspnea each day in the prior 24 hours usually occurred at rest. Dyspnea declined but persisted through hospitalization for most patients. “Air hunger” was the dominant sensation, especially when dyspnea was strong (>4). Anxiety and frustration were the dominant emotions associated with dyspnea.
Conclusions
This first multidimensional portrait of dyspnea in a general inpatient population characterizes the sensations and emotions dyspneic patients endure. The finding that air hunger is the dominant sensation of severe dyspnea has implications for design of laboratory models of these sensations and may have implications for targets of palliation of symptoms.
Key Words: dyspnea, hospitalized patients, inpatients, symptoms
Abbreviations: A1, overall breathing discomfort; MDP, Multidimensional Dyspnea Profile; SD, study day; SQ, sensory quality
Moderate to severe dyspnea (rating ≥4/10) has been estimated to affect between 4% and 10% of hospitalized patients, approximately one-half as prevalent as moderate to severe pain in the same group.1, 2, 3 We have been unable to find published information on the sensory or emotional experience of dyspnea in these patients, however. We describe a multidimensional profile of dyspnea in hospitalized patients in an effort to help guide research into symptom management strategies and to provide health-care providers with better information about their patients’ experiences.
The most commonly used clinical dyspnea instruments, reviewed in Bausewein and colleagues4, Dorman and colleagues5, and Johnson and colleagues,6 do not ask the patient to rate or describe his or her dyspnea, rather, they ask patients to recall which activities have been limited by dyspnea (eg, the Medical Research Council breathlessness scale, the Baseline/Transitional Dyspnea Index).7, 8 Such instruments provide useful functional data to assess patients; however, most of these instruments are framed to ask about days or weeks of dyspnea during usual activities and would not make semantic sense if used to assess dyspnea at a specific time or during a specific activity, for instance current dyspnea in a hospitalized patient, or worst dyspnea during an exercise test. Most experimental studies of dyspnea and a limited number of clinical studies in acute settings have used rating scales to measure dyspnea at a specific time using visual analog scales,9, 10, 11 or numerical rating scale,12, 13, 14 or various modifications of Borg scales15, 16, 17, 18, 19; however, these rating scales have treated dyspnea as a single dimension.
The concept that pain comprises multiple dimensions, proposed many years ago, was a breakthrough in the understanding of pain20, 21, 22, 23 and is now essential to “state-of-the-art” pain science and pain management.24, 25 The multidimensional concept has more recently been applied to dyspnea: there is evidence demonstrating that different sensory qualities of breathlessness are connected to specific physiological mechanisms,26, 27, 28, and that dyspnea evokes several emotional responses, such as anxiety and depression.29, 30, 31 This evidence led to the development of the most widely cited definition of dyspnea: “a subjective experience of breathing discomfort that consists of qualitatively distinct sensations that vary in intensity… distinct mechanisms and afferent pathways are reliably associated with different sensory qualities (notably work/effort, tightness, and air hunger/unsatisfied inspiration) [that] most often do not occur in isolation [and] vary in their unpleasantness and in their emotional and behavioral significance.”1, 32, 33 The Multidimensional Dyspnea Profile (MDP) is a validated instrument designed by our group to provide a profile of the several sensory and emotional components of dyspnea.34 Another instrument incorporating the multidimensional concept is the “Dyspnea 12,” which was designed primarily to provide a single global score from a composite of items that include both sensory and emotional components of dyspnea. The similarities and differences between these instruments have been discussed at length 35, 36 and data comparing their use in ambulatory COPD patients have been published.37
We endeavored to provide a broad description of the experience of dyspnea across diagnoses as health-care providers confront it in the hospital. Using the MDP3 to characterize these qualitatively distinct sensory qualities and emotional responses associated with dyspnea, we describe dyspnea among patients hospitalized at a single tertiary care facility. We hypothesized that patients’ experience of dyspnea, including their assessment of the intensity of dyspnea, description of the associated sensations, and emotional experience of dyspnea, would vary before hospitalization and over the course of the hospital stay. Understanding the most common sensations and emotional responses can help guide health-care providers because they manage the symptom while treating the underlying disease process, and it may assist researchers when designing experiments intended to reflect the clinical situation.
Materials and Methods
Setting and Study Population
We studied patients older than age 18 admitted between January and August 2014 at a single academic medical center in Boston, MA, regardless of underlying diagnosis. Dyspnea is documented as part of routine care in all medical-surgical units at our institution, using a 0 to 10 scale (with 10 anchored at “unbearable”). We attempted to identify and recruit all patients who rated dyspnea 4 or greater as documented by the patient’s nurse on the day of admission and who could communicate in English; these data were not included in the study but were used for admission criteria alone. Data for this study were then collected by research staff after admission and daily thereafter when possible. The first research interview occurred as soon as possible after the patient arrived to the inpatient unit. A total of 84% patients in this cohort arrived on the medical-surgical unit after spending time in the ED. Practical limitations on recruitment, retention, and data collection are detailed in the supplemental material (e-Appendixes 1-6 provide greater detail on how the study was conducted. e-Appendix 7 is the detailed protocol. e-Figure 1 provides a consort diagram of the study. e-Table 1 provides the number of patients enrolled by hospital day, and e-Table 2 includes patient characteristics of the cohort studied. e-Figure 2 compares first vs worst dyspnea ratings. e-Figure 3 demonstrates the highly variable patterns of dyspnea over time. e-Figure 4 compares pain ratings and acceptability). Data were neither entered in the medical record nor communicated to clinical staff. Our study was approved by the institutional review board at the Beth Israel Deaconess Medical Center (2013P000268) and verbal informed consent was obtained from all participants; patients were informed that interviews were for research and not of direct benefit to them.
Dyspnea Assessment
Research staff attempted to visit all participating patients and conduct a research interview once per day throughout their hospitalizations to administer the MDP (Table 1), an instrument that uses numeric rating scales for three dimensions of dyspnea. The development and validation of this instrument is described elsewhere.34, 36, 37 Diagram and protocol of patients enrolled and excluded is available in supplemental materials (e-Fig 1, e-Appendix 1). Participants were asked to complete the A1 scale (overall breathing discomfort) for both (1) their current dyspnea at the time of the interview and (2) their worst dyspnea in the past 24 hours; 86 patients completed both and 70 completed one. We used several strategies to group patients for the analyses shown below. Study day 1 (SD1) was considered the first day on which data were collected; because of loss to follow-up, day 1 is the day with maximum participants, and each participant is represented once, therefore this grouping was used for cross-sectional analyses in Figures 1 and 4. There were 64 patients who provided data on day 1 and day 2; this group was used to examine temporal change in Figure 2. The maximum sample is 460 MDPs completed by all patients on all study days, but this sample weights patients who participated for more hospital days more heavily; these data were used for Figure 3.
Table 1.
Elements of MDP
| MDP Elements | Scale |
|---|---|
| 1. A1: The unpleasantness or discomfort of breathing sensations | 0-10 where 0 = neutral, 10 = unbearable |
| 2. SQ force choice | Pick one sensory quality that describes breathing discomfort and intensity of sensory qualities |
| 3. SQ rating | For each item, provide a rating 0-10 where 0 = none, 10 = as intense as I can imagine |
| Effort | |
| Air hunger | |
| Tightness | |
| Mental effort | |
| Breathing a lot | |
| Other | |
| 4. Intensity of five negative emotions | For each item, provide a rating 0-10 where 0 = none, 10 = the most I can imagine |
| Depressed | |
| Anxious | |
| Frustrated | |
| Angry | |
| Afraid | |
| Other |
MDP = Multidimensional Dyspnea Profile; SQ = sensory quality.
Figure 1.
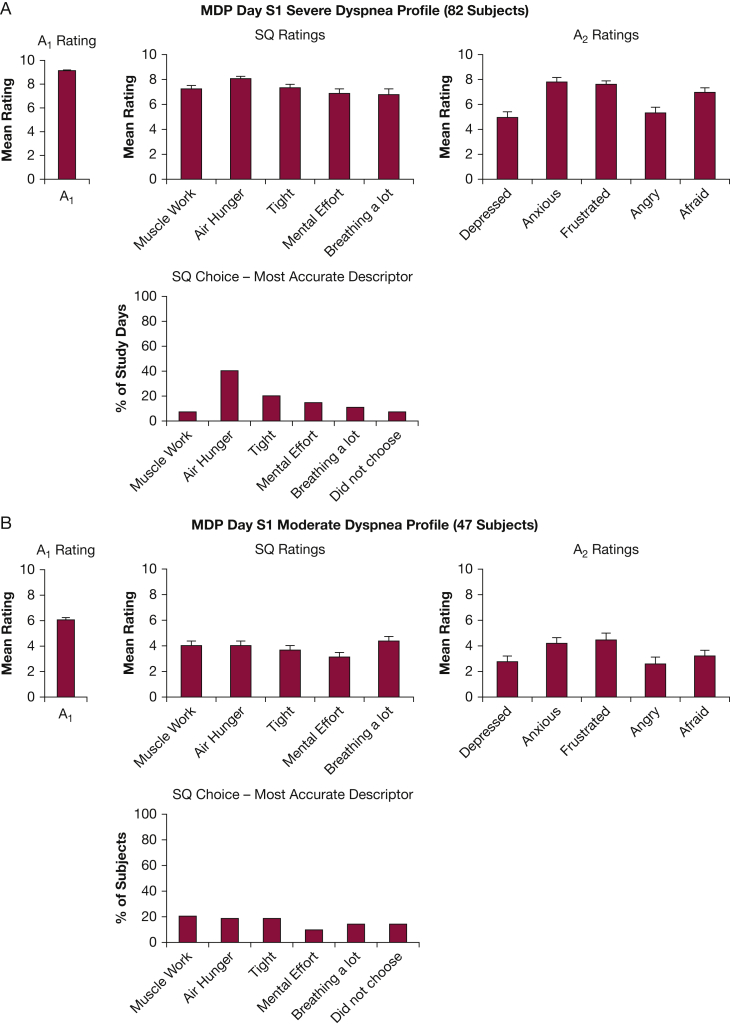
Multidimensional profile of 129 subjects describing breathing discomfort associated with their highest A1 rating on study day 1; either their dyspnea in the past 24 hours or their current dyspnea, whichever was worst. Columns depict mean ± standard error. Each patient contributed one MDP; thus, patients are given equal weight, and this is a cross-sectional comparison. A, A1 rating of 8.0 to 10.0 was classed as severe dyspnea (82/129 subjects). B, An A1 rating of 4.0 to 7.9 was classed as moderate dyspnea (47/129 subjects). Five additional patients provided a completed ‘worst’ dyspnea profile for this day, but rated A1 < 4. The remainder of patients (23) did not provide a full profile on the initial day. A1 = overall breathing discomfort; A2 = emotional responses to dyspnea; MDP = Multidimensional Dyspnea Profile; SQ = sensory quality.
Figure 4.
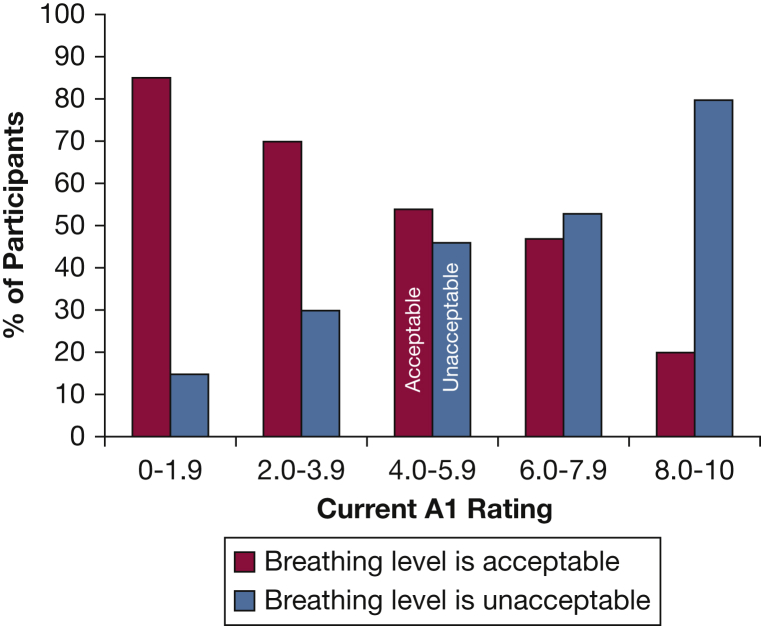
Plot of acceptability of breathing discomfort vs current A1 rating on study day 1 (n = 137 participants who provided data on acceptability). See Figure 1 legend for expansion of abbreviation.
Figure 2.
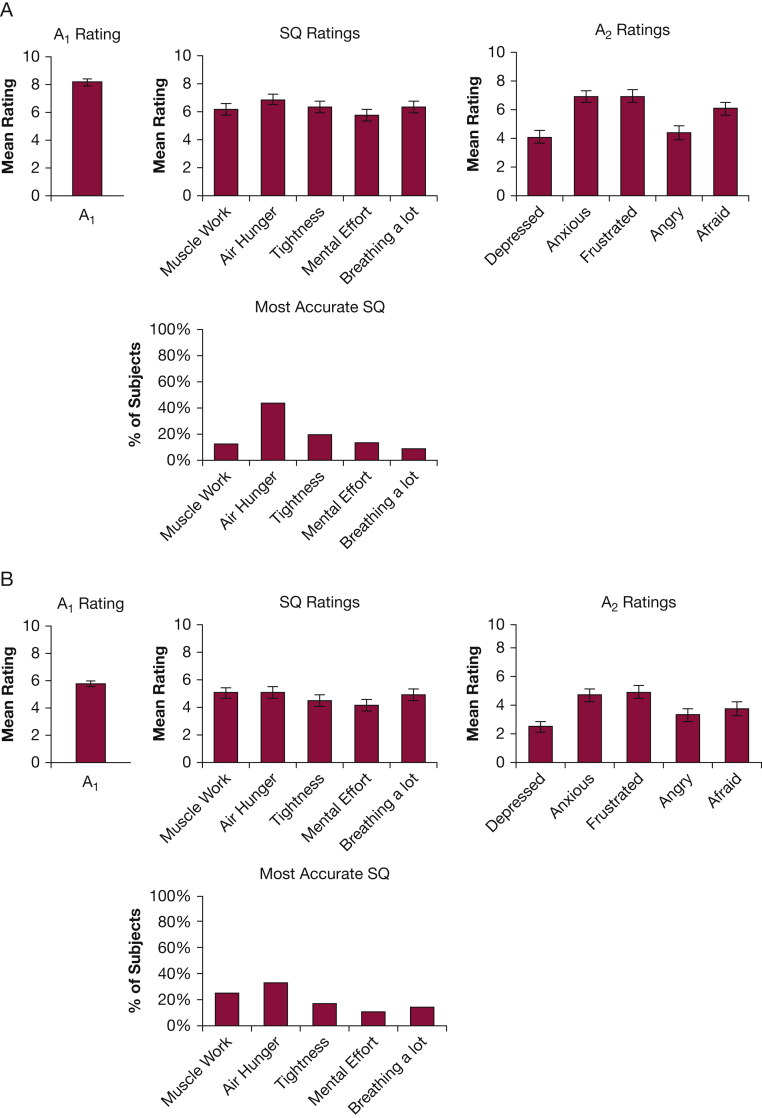
Progression of dyspnea with time for patients with two consecutive study days. Sixty-four patients provided full MDP data for “worst breathing discomfort in past day” on both study days 1 and 2. A, The average profile of these patients on study day 1 and (B) the profile of the same patients on study day 2. Mean A1 fell from 8.1 to 5.8, and all ratings fell, but not equally; air hunger became clearly less prominent at lower dyspnea levels. It is notable that these profile differences for high and moderate A1 within-group are very similar to those in the across-groups comparison shown in Figure 1. See Figure 1 legend for expansion of abbreviations.
Figure 3.
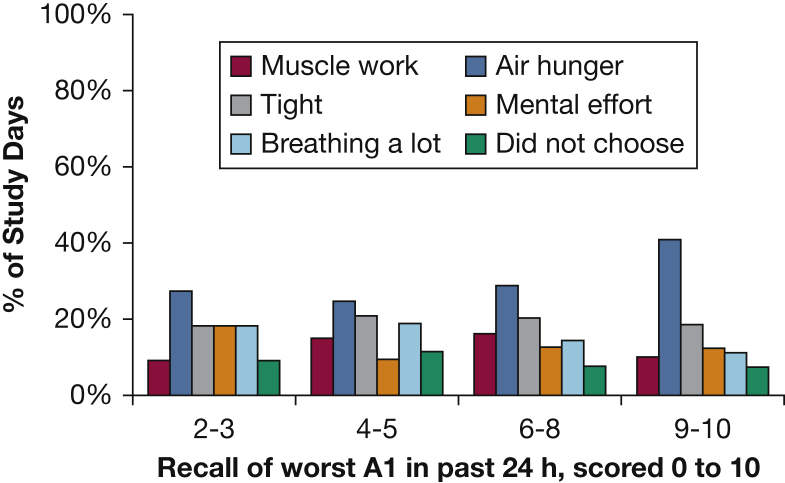
Profile of all 460 subject responses across all study days when asked to choose the sensory quality that most accurately describes their worst dyspnea in the past 24 hours. Subjects who remained in hospital longer contributed more data; thus, this graph would represent prevalence in the hospital on any day, not prevalence among individuals (as presented in Fig 1). On the x-axis, half ratings for A1 are rounded down and are included in the appropriate cluster (ie, an A1 rating of 5.5 will be included in the 4-5 grouping). There are 22 study days in the A1 = 2-3 group, 53 study days in the A1 = 4-5 group, 118 study days in the A1 = 6-8 group, and 81 subjects study days in the A1 = 9-10 group. See Figure 1 legend for expansion of abbreviation.
We also asked patients (N = 138), “Is your breathing discomfort at an acceptable level now?” to provide data on which to base symptom management guidelines. Patients were also about the acceptability of their pain.
Other Study Variables
We obtained demographic information, including age, race, and sex, discharge diagnoses, and comorbidities for each patient from the electronic health record; these data were not available for patients classified as “observation” status.
Statistical Analysis
All statistical tests were performed using SAS (v. 9.4, SAS Institute Inc., Cary, NC). Descriptive statistics were summarized and compared using Student t test, χ2, or Fisher exact tests when cell sizes were small, as appropriate. In the case of multiple comparisons, a Bonferroni correction was performed. Data examined over time were clustered within patient, and weighted on the basis of the number of daily measurements. In several cross-sectional analyses we present SD1 data because this sample is equally weighted for each subject and because it contains the largest number of subjects.
Results
Cohort Description
We identified and approached 267 patients who met criteria during a 7-month period; 156 consented to participate (Consort diagram: e-Fig 1). During this time, 27,473 patients were admitted to our institution to medical-surgical floors. On average, patients participated for 2.6 days (minimum 1, maximum 12); 88% of the patients provided data within 1 day of admission. Of the 156 patients, 147 completed at least one MDP for the worst dyspnea in 24 hours, 142 patients completed at least one MDP in which overall breathing discomfort (A1) was rated ≥4. The MDP was completed by 140 patients on the first study day, SD1 (16 agreed to participate, but postponed the first full interview). There were 460 completed MDPs, and an additional 30 ratings of A1 alone.
Of the 156 patients, 13 were designated as “observation status” rather than formal admission. Among the 143 inpatients who were admitted, the average length of stay was 6 days; 18 patients were admitted to the ICU (mean ICU length of stay: 3 days). The most common discharge diagnoses are shown in Table 2. Eight of the 156 patients (5.1%) died (compared with the hospital-wide mortality rate of 1.1% for the same time period; e-Table 2). Fifty-six percent (87 of 156) of the cohort were women, 66% (103 of 156) self-reported “white,” and the mean age was 63 (SD 16 years).
Table 2.
Common Discharge Diagnoses
| Diagnoses | No. (%) |
|---|---|
| Chronic diastolic heart failure | 14 (9.0) |
| Pneumonia | 11 (7.1) |
| COPD with exacerbation | 11 (7.1) |
| Acute chronic systolic heart failure | 7 (4.5) |
| Sepsis | 4 (2.6) |
| Asthma | 4 (2.6) |
| Acute kidney injury | 4 (2.6) |
Overall Breathing Discomfort (A1)
There was a substantial difference between dyspnea reported at the study interview and the worst dyspnea recalled in the past 24 hours. We compared these two ratings by patients on the first study day because there were more individuals responding on that day. On the first study day, patients rated current dyspnea A1 a mean of 4.3 (SD = 2.2; 146 subjects), whereas the average worst A1 in the past 24 hours was 7.8 (SD = 2.3; 138 subjects, P < .001). On this first day of enrollment, we found that A1 = 10 was the most common rating for worst dyspnea in the past day (41 of 138 patients). In contrast, hours later, no participant rated their dyspnea at the time of interview as a 10 (e-Fig 2). In 161 study interviews, information was collected about the activity associated with worst dyspnea; the most common were: rest (47%), minor exertion (34%), coughing (4%), and changes in posture (3%). Worst dyspnea was occasionally associated with nebulizer treatments, pain, anxiety, talking, and eating.
Most patients’ dyspnea decreased over subsequent study interviews, although patients described many temporal patterns of dyspnea (e-Fig 3). Twenty-two patients reported at least one study day on which their worst dyspnea was the same or worse than the previous study day.
Profiles of Dyspnea: Sensory Qualities and Emotional Responses to Dyspnea
Air hunger was the most prominent sensation reported by patients, especially those with higher levels of dyspnea. The most prominent emotions associated with dyspnea were anxiety and frustration. We used two approaches to examine the relation of overall breathing discomfort (A1 rating) to the profile of sensations and emotions: (1) comparison of groups of patients with different A1 ratings on SD1, and (2) comparison of successive days in the same patients as treatment progressed and A1 decreased.
Profile Comparison Across Patients on SD1
We compared the dyspnea profile on the first study day for those patients with moderate dyspnea in the past 24 hours (A1 rating 4-7.9, N = 47) to the dyspnea profile of patients who reported severe dyspnea (A1 rating 8-10, N = 82) in Figure 1.
At moderate dyspnea levels (Fig 1B), neither the sensory quality (SQ) ratings nor the SQ forced choice revealed a strong distinction among sensory qualities of dyspnea. It is notable, however, that even with moderate dyspnea, patients reported more frustration and anxiety (Figure 1, Figure 2).
The intensity of all sensory qualities was higher in patients reporting high overall discomfort (A1); air hunger ratings were somewhat higher than other sensations (P < .001, adjusted for multiple comparisons with a Bonferroni correction). Distinctions among sensations were sharpened when subjects were required to choose which sensation most accurately described their breathing: at high A1 levels, air hunger was chosen as the most apt descriptor more frequently than other sensory qualities (P = .02; Fig 3). Anxiety and frustration became more prominent and fear emerged in patients with more severe dyspnea (P < .001 for each pairwise comparison; Figure 1, Figure 2).
Profile Comparison Within Subject Across SD1 and SD2
We examined the change in profile within patients when dyspnea declined with time. We selected the 64 subjects who fully completed MDPs on both SD1 and SD2. We compared their profiles on SD1 (mean A1 = 8.1) to their profiles on SD2 (mean A1 = 5.8) (Fig 2). The sensory and emotional profile characteristics for high and moderate A1 in this within-subject comparison are very similar to the across-subject profile comparison shown in Figure 1.
Acceptable Levels of Dyspnea
We examined the relationship between the current dyspnea ratings of patients on SD1 and whether they felt that symptom level to be acceptable or unacceptable. Patients who felt their current dyspnea was acceptable had a significantly lower mean A1 than patients who felt their current dyspnea to be unacceptable (3.7 vs 5.2, P < .0001), as would be expected.
Among the 48 patients who rated current A1 <4/10 with research staff on the first study day, 75% found their current breathing discomfort to be acceptable. Conversely, among the 90 patients who rated their current A1 > 4 on the first day, 57% found their dyspnea to be unacceptable (Fig 4). For comparison, information about acceptability of pain are presented in the supplemental materials (e-Fig 4).
Discussion
This is the first multidimensional description of dyspnea among general hospitalized medical-surgical patients. Our principal findings are: (1) air hunger was the predominant sensation, especially in severe dyspnea; (2) frustration, anxiety, and fear were the dominant emotions during moderate and severe dyspnea; (3) there was a strong relationship between the overall level of dyspnea and the average profile of sensory qualities and emotions that can serve as a guide in interpreting individual patient profiles; (4) assessments of current dyspnea at a time of convenience are likely to miss the worst dyspnea of the day; (5), our data show wide individual variation in the level of dyspnea that patients find acceptable, suggesting that symptom control measures should be on the basis of individual patient preference; however, our data are consistent with using a rating of 4/10 as a rough guide for assessing the adequacy of symptom control in an institution or unit.38
Air Hunger Is the Principle Component of Severe Dyspnea
The sensory quality of respiratory discomfort provides a clue to the underlying neurophysiological mechanism in play.1, 39 Understanding the dominant sensation can provide insights when choosing laboratory models to approximate the patient’s experience, and may guide symptomatic therapy. We found several uncomfortable sensations contribute to discomfort at low to moderate levels of dyspnea; however, when patients reported severe dyspnea, air hunger was chosen more than twice as frequently as tightness or respiratory muscle effort. Similar dominance of air hunger (or “unsatisfied inspiration”1) has been reported in studies of outpatients using the MDP and other instruments40, 41, 42 but is not a universal finding.37, 43 The emergence of air hunger at high discomfort levels in these inpatients is consistent with laboratory experiments in which even moderate air hunger was more unpleasant and produced more fear and anxiety than maximal respiratory work.44 Most notably, air hunger, the most common sensation we observed during moderate to severe dyspnea arises when tidal volume and ventilation (as sensed by mechanoreceptors, mainly pulmonary stretch receptors45, 46) is less than respiratory demand (as sensed from corollary discharge from brainstem respiratory centers47, 48). Air hunger can be addressed by actions taken to increase ventilation (eg, coaching more effective breathing patterns, reducing bronchoconstriction with bronchodilators, or mechanical ventilatory support) or by actions taken to reduce respiratory drive (eg, opiates).
Other Dyspnea Sensations
Although air hunger was the dominant sensation reported, a substantial number of patients chose other sensory descriptors such as tightness, respiratory muscle work. Tightness is usually associated with bronchoconstriction, and probably arises from pulmonary afferents,49, 50 and can be ameliorated by bronchodilation.51 A sense of excessive respiratory work and effort is thought to arise from both respiratory muscle afferents and from an awareness of cortical motor command52, 53; respiratory work can be diminished by providing assistance to breathing via mechanical support or by reducing the drive to breathe (both of which are also likely to diminish air hunger). Patients also sometimes reported an awareness of increased breathing and a need to concentrate on breathing: these are not uncomfortable per se, but may contribute to anxiety and frustration.
Emotional Response
The dominant emotions related to breathing discomfort were anxiety, frustration, and fear; these emotions were present even at moderate levels of dyspnea. These emotions are also dominant in laboratory dyspnea challenges intended to evoke air hunger and in ambulatory patients with COPD.44, 54 For a given level of discomfort, we found ratings of anxiety to be higher in our inpatients than in outpatients with COPD, in healthy people experiencing air hunger in the laboratory or experiencing exertion-related dyspnea in daily life.34, 54 Medical-surgical inpatients in this study were as anxious as ED patients at the time of decision to go to the hospital.55
Worst Dyspnea in the Past 24 Hours Was Usually Substantially Greater Than Current Dyspnea at Interview
Patients in this study usually experienced severe dyspnea sometime during each day, which would have been missed by our daily point assessment of current dyspnea. Very few patients described their current dyspnea at the time of interview as unbearable or nearly unbearable (8-10/10). Yet, ratings of 8 to 10 were the most prevalent response when patients were asked to recall their worst dyspnea in the past 24 hours. Recall of recent dyspnea using the MDP is reliable.34 Although the episodic nature of dyspnea in our subjects was often due to activities of care (eg, with movement to the bathroom or working with physical therapy), in one-half of the instances, patients experienced the worst dyspnea at rest. These severe episodes of dyspnea may be missed by nursing assessments of dyspnea once or twice per day; nursing assessment of recalled as well as current dyspnea may be necessary to understand and manage the symptom burden in these patients.56, 57 In particular, there may be value in evaluating dyspnea more frequently in patients who report higher symptom burdens, particularly when that report is in the past 24 hours while hospitalized.
How Much Dyspnea Is Too Much?
Dyspnea rating > 4 has been considered to be meaningful discomfort on the basis of prior studies and evaluations in the pain literature.2, 38 More than one-half of patients who rated overall breathing discomfort > 4 deemed that level of breathing discomfort unacceptable. A minimum rating of 4/10 of breathing discomfort obtained by the nurse on admission of was required for entry into the study, but at the time of our interview several hours later current overall breathing discomfort ratings ranged from 0 to 9. Even at ratings above 6, 5% of the patients still deemed their breathing discomfort acceptable, whereas 6% of patients rating 2 or less deemed the discomfort unacceptable; this highlights the substantial variability of the individual experience of dyspnea. Although a rating of 4 appears to be an acceptable benchmark for judging dyspnea management at the unit or hospital level, the wide variability suggests to us that each patient who reports any dyspnea should be asked if their level of dyspnea is acceptable or requires management. A wide range of pharmacologic and non-pharmacologic symptom management strategies are available.1
Do These Results Provide Guidance for Improved Dyspnea Assessment and Management?
Unidimensional measurement of dyspnea both on admission and throughout hospitalization is feasible and adequate for routine screening.2, 58 Our data identify air hunger as the dominant discomfort among patients with severe dyspnea, so air hunger should be a target of symptom relief strategies. Anxiety was the most prominent emotion, consistent with other findings that air hunger is preeminent among respiratory sensations in provoking anxiety; healthy subjects undergoing laboratory air hunger report anxiety despite knowing they are safe, and that they can terminate the discomfort instantly.44, 54 This primal emotional link is a normal response to air hunger, and reducing the strength of this link may be a target of intervention, especially in those patients who have anxiety disproportionate to their discomfort.
These results also highlight the utility of an instrument, the MDP, for clinically investigating an individual patient’s dyspnea further, when a health-care provider encounters a patient reporting high levels of discomfort or burden from the symptom. In individual patients who do not respond to palliative efforts, or who do not have an adequate diagnosis, the MDP may be useful for follow-up questioning, especially if used by a resource nurse or physician with some added training in dyspnea assessment and management. The mean profiles we provide for different levels of overall discomfort provide a basis of comparison for individual patients; large departures from the mean profile (eg, a breathing-related anxiety level of 9 with overall breathing discomfort of 4) could highlight issues for further investigation. A discussion of the use of the MDP for research and clinical care is discussed further in the supplemental materials (e-Appendixes 2-7).
Study Limitations
First, although our response rate on the day of admission to the study was robust, our subsequent daily response rate was more modest (because of both patient discharge and patient dropout), limiting our ability to describe the time course of dyspnea.
Second, our objective was to profile dyspnea among the full spectrum of dyspneic hospitalized patients; because of this, broad inclusion our ability to further characterize the patterns of dyspnea in patients with particular diseases is limited.
Third, although we instructed patients to rate the emotions related to their breathing discomfort, those emotions could be confounded by patients’ overall emotional response to illness, hospitalization, and treatment. The strong relationship of emotion to concurrent dyspnea A1 rating suggests that, for the most part, patients did focus on emotions related to breathing.
Fourth, because of logistical obstacles, we were unable to assess patients when they arrived at the hospital to determine the effect of early treatment prior to admission, as detailed in Methods and the supplemental methods.
Fifth, our study was not designed to determine a definitive threshold for treatment of dyspnea; we asked only one question: is this level of dyspnea acceptable? We did not ask further questions about whether treatment was desired, and what treatment side effects or other costs would be acceptable. Prior authors have suggested a benchmark of 4 for palliation of pain and dyspnea, our work suggests that health-care providers realize there is wide variation among patients’ experience of dyspnea.7
Conclusions
We studied the broad spectrum of dyspneic medical-surgical hospitalized patients on multiple days during their hospitalization. Air hunger was the dominant sensation in severe dyspnea, and anxiety, frustration, and fear were the dominant emotions. Air hunger is accompanied by substantial levels of respiratory work, tightness, and awareness of “breathing a lot,” which can help shape symptom management strategies and laboratory dyspnea models. The worst dyspnea recalled from the past day was often severe; patients should be asked about their experience since the last assessment. These preliminary “normative” profiles will help interpret individual inpatients’ responses to the MDP, and provide guidance for future research.
Acknowledgments
Author contributions: J. P. S. and R. B. B. conducted the study, reviewed and analyzed the data, wrote the manuscript, and meaningfully revised it. R. M. S., R. W. L., and K. B. meaningfully guided the study and meaningfully revised the manuscript. A. R. S. analyzed the data and revised the manuscript. H. B. B. conducted the interviews, analyzed the data, and revised the manuscript.
Financial/nonfinancial disclosures: None declared.
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Other contributions: The authors thank Victoria (Hatch) Harrington, Heather Bernstein, Dora Huang, and Victoria Molina for skillfully interviewing patients and recording data; the Beth Israel Deaconess Medical Center nursing staff for cooperation and assistance; Carl O’Donnell for helping us solve many logistical problems, and for thoughtful commentary on our data; and most important, we thank the patients who participated in this study for their time and cooperation.
Additional information: The e-Appendixes, e-Figures, and e-Tables can be found in the Supplemental Materials section of the online article.
Footnotes
FUNDING/SUPPORT: This study was funded by the National Institutes of Health [Grant NR010006]. J. P. S. is also supported by grant number K08HS024288 from the Agency for Healthcare Research and Quality. The content is solely the responsibility of the authors and does not represent the official views of the Agency for Healthcare Research and Quality. J. P. S. is also supported by a grant from the Doris Duke Charitable Foundation.
Supplementary Data
References
- 1.Parshall M.B., Schwartzstein R.M., Adams L. An official American Thoracic Society statement: update on the mechanisms, assessment, and management of dyspnea. Am J Respir Crit Care Med. 2012;185(4):435–452. doi: 10.1164/rccm.201111-2042ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stevens J.P., Baker K., Howell M.D., Banzett R.B. Prevalence and predictive value of dyspnea ratings in hospitalized patients: pilot studies. PLoS One. 2016;11(4) doi: 10.1371/journal.pone.0152601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stevens J.P., Dechen T., Schwartzstein R. Prevalence of dyspnea among hospitalized patients at the time of admission. J Pain Symptom Manage. 2018;56(1):15–22. doi: 10.1016/j.jpainsymman.2018.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bausewein C., Booth S., Higginson I.J. Measurement of dyspnoea in the clinical rather than the research setting. Curr Opin Support Palliat Care. 2008;2(2):95–99. doi: 10.1097/SPC.0b013e3282ffafe8. [DOI] [PubMed] [Google Scholar]
- 5.Dorman S., Byrne A., Edwards A. Which measurement scales should we use to measure breathlessness in palliative care? A systematic review. Palliat Med. 2007;21(3):177–191. doi: 10.1177/0269216307076398. [DOI] [PubMed] [Google Scholar]
- 6.Johnson M.J., Oxberry S.G., Cleland J.G., Clark A.L. Measurement of breathlessness in clinical trials in patients with chronic heart failure: the need for a standardized approach: a systematic review. Eur J Heart Fail. 2010;12(2):137–147. doi: 10.1093/eurjhf/hfp194. [DOI] [PubMed] [Google Scholar]
- 7.Fletcher C. The clinical diagnosis of pulmonary emphysema - an experimental study. Proc Res in Social Med. 1952;45(9):577–584. [PubMed] [Google Scholar]
- 8.Mahler D.A., Weinberg D.H., Wells C.K., Feinstein A.R. The measurement of dyspnea. Contents, interobserver agreement, and physiologic correlates of two new clinical indexes. Chest. 1984;85(6):751–758. doi: 10.1378/chest.85.6.751. [DOI] [PubMed] [Google Scholar]
- 9.Aitken R.C. Measurement of feelings using visual analogue scales. Proc R Soc Med. 1969;62(10):989–993. [PMC free article] [PubMed] [Google Scholar]
- 10.Gift A.G. Validation of a vertical visual analogue scale as a measure of clinical dyspnea. Rehabil Nurs. 1989;14(6):323–325. doi: 10.1002/j.2048-7940.1989.tb01129.x. [DOI] [PubMed] [Google Scholar]
- 11.Adams L., Chronos N., Lane R., Guz A. The measurement of breathlessness induced in normal subjects: validity of two scaling techniques. Clin Sci (Lond) 1985;69(1):7–16. doi: 10.1042/cs0690007. [DOI] [PubMed] [Google Scholar]
- 12.Gift A.G., Narsavage G. Validity of the numeric rating scale as a measure of dyspnea. Am J Crit Care. 1998;7(3):200–204. [PubMed] [Google Scholar]
- 13.Saracino A., Weiland T., Dent A., Jolly B. Validation of a verbal dyspnoea rating scale in the emergency department. Emerg Med Australas. 2008;20(6):475–481. doi: 10.1111/j.1742-6723.2008.01132.x. [DOI] [PubMed] [Google Scholar]
- 14.Morris N.R., Sabapathy S., Adams L., Kingsley R.A., Schneider D.A., Stulbarg M.S. Verbal numerical scales are as reliable and sensitive as visual analog scales for rating dyspnea in young and older subjects. Respir Physiol Neurobiol. 2007;157(2-3):360–365. doi: 10.1016/j.resp.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 15.Borg E., Borg G., Larsson K., Letzter M., Sundblad B.M. An index for breathlessness and leg fatigue. Scand J Med Sci Sports. 2010;20(4):644–650. doi: 10.1111/j.1600-0838.2009.00985.x. [DOI] [PubMed] [Google Scholar]
- 16.Borg G.A. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14(5):377–381. [PubMed] [Google Scholar]
- 17.Kendrick K.R., Baxi S.C., Smith R.M. Usefulness of the modified 0-10 Borg scale in assessing the degree of dyspnea in patients with COPD and asthma. J Emerg Nurs. 2000;26(3):216–222. doi: 10.1016/s0099-1767(00)90093-x. [DOI] [PubMed] [Google Scholar]
- 18.Supinski G., Dimarco A., Bark H., Chapman K., Clary S., Altose M. Effect of codeine on the sensations elicited by loaded breathing. Am Rev Respir Dis. 1990;141(6):1516–1521. doi: 10.1164/ajrccm/141.6.1516. [DOI] [PubMed] [Google Scholar]
- 19.O'Donnell D.E., Webb K.A. Exertional breathlessness in patients with chronic airflow limitation. The role of lung hyperinflation. Am Rev Respir Dis. 1993;148(5):1351–1357. doi: 10.1164/ajrccm/148.5.1351. [DOI] [PubMed] [Google Scholar]
- 20.Dallenbach K. Somesthesis. In: Boring E.G., Langfield H.S., Weld H.P., editors. Introduction to Psychology. Wiley and Sons; New York: 1939. pp. 608–625. [Google Scholar]
- 21.Wade J.B., Dougherty L.M., Archer C.R., Price D.D. Assessing the stages of pain processing: a multivariate analytical approach. Pain. 1996;68(1):157–167. doi: 10.1016/S0304-3959(96)03162-4. [DOI] [PubMed] [Google Scholar]
- 22.Price D.D. Psychological and neural mechanisms of the affective dimension of pain. Science. 2000;288(5472):1769–1772. doi: 10.1126/science.288.5472.1769. [DOI] [PubMed] [Google Scholar]
- 23.Melzack R. The McGill Pain Questionnaire: major properties and scoring methods. Pain. 1975;1(3):277–299. doi: 10.1016/0304-3959(75)90044-5. [DOI] [PubMed] [Google Scholar]
- 24.Dworkin R.H., Turk D.C., Farrar J.T. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain. 2005;113(1,Äì2):9–19. doi: 10.1016/j.pain.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 25.Piotrowski C. Assessment of pain: a survey of practicing clinicians. Percept Mot Skills. 1998;86(1):181–182. doi: 10.2466/pms.1998.86.1.181. [DOI] [PubMed] [Google Scholar]
- 26.Elliott M.W., Adams L., Cockcroft A., MacRae K.D., Murphy K., Guz A. The language of breathlessness. Use of verbal descriptors by patients with cardiopulmonary disease. Am Rev Respir Dis. 1991;144(4):826–832. doi: 10.1164/ajrccm/144.4.826. [DOI] [PubMed] [Google Scholar]
- 27.Simon P.M., Schwartzstein R.M., Weiss J.W. Distinguishable sensations of breathlessness induced in normal volunteers. Am. Rev. Respir. Dis. 1989;140(4):1021–1027. doi: 10.1164/ajrccm/140.4.1021. [DOI] [PubMed] [Google Scholar]
- 28.Simon P.M., Schwartzstein R.M., Weiss J.W., Fencl V., Teghtsoonian M., Weinberger S.E. Distinguishable types of dyspnea in patients with shortness of breath. Am Rev Respir Dis. 1990;142(5):1009–1014. doi: 10.1164/ajrccm/142.5.1009. [DOI] [PubMed] [Google Scholar]
- 29.Oswald N.C., Waller R.E., Drinkwater J. Relationship between breathlessness and anxiety in asthma and bronchitis: a comparative study. Br Med J. 1970;2(5700):14–17. doi: 10.1136/bmj.2.5700.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carrieri-Kohlman V., Gormley J.M., Douglas M.K., Paul S.M., Stulbarg M.S. Exercise training decreases dyspnea and the distress and anxiety associated with it. Monitoring alone may be as effective as coaching. Chest. 1996;110(6):1526–1535. doi: 10.1378/chest.110.6.1526. [DOI] [PubMed] [Google Scholar]
- 31.Gift A.G. Psychologic and physiologic aspects of acute dyspnea in asthmatics. Nurs Res. 1991;40(4):196–199. [PubMed] [Google Scholar]
- 32.ATS ad hoc Committee Dyspnea. Mechanisms, assessment, and management: a consensus statement. American Thoracic Society. Am J Respir Crit Care Med. 1999;159(1):321–340. doi: 10.1164/ajrccm.159.1.ats898. [DOI] [PubMed] [Google Scholar]
- 33.Laviolette L., Laveneziana P. Dyspnoea: a multidimensional and multidisciplinary approach. Eur Respir J. 2014;43(6):1750–1762. doi: 10.1183/09031936.00092613. [DOI] [PubMed] [Google Scholar]
- 34.Parshall M.B., Meek P.M., Sklar D., Alcock J., Bittner P. Test-retest reliability of multidimensional dyspnea profile recall ratings in the emergency department: a prospective, longitudinal study. BMC Emerg Med. 2012;12:6. doi: 10.1186/1471-227X-12-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Banzett R.B., Moosavi S.H. Measuring dyspnoea: new multidimensional instruments to match our 21st century understanding. Eur Respir J. 2017;49(3) doi: 10.1183/13993003.02473-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Banzett R.B., O'Donnell C.R., Guilfoyle T.E. Multidimensional dyspnea profile: an instrument for clinical and laboratory research. Eur Respir J. 2015;45(6):1681–1691. doi: 10.1183/09031936.00038914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Williams M.T., John D., Frith P. Comparison of the Dyspnoea-12 and Multidimensional Dyspnoea Profile in people with COPD. Eur Respir J. 2017;49(3) doi: 10.1183/13993003.00773-2016. [DOI] [PubMed] [Google Scholar]
- 38.Twaddle M.L., Maxwell T.L., Cassel J.B. Palliative care benchmarks from academic medical centers. J Palliat Med. 2007;10(1):86–98. doi: 10.1089/jpm.2006.0048. [DOI] [PubMed] [Google Scholar]
- 39.Lansing R.W., Gracely R.H., Banzett R.B. The multiple dimensions of dyspnea: review and hypotheses. Respir Physiol Neurobiol. 2009;167(1):53–60. doi: 10.1016/j.resp.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chowienczyk S., Javadzadeh S., Booth S., Farquhar M. Association of descriptors of breathlessness with diagnosis and self-reported severity of breathlessness in patients with advanced chronic obstructive pulmonary disease or cancer. J Pain Symptom Manage. 2016;52(2):259–264. doi: 10.1016/j.jpainsymman.2016.01.014. [DOI] [PubMed] [Google Scholar]
- 41.Smith J., Albert P., Bertella E., Lester J., Jack S., Calverley P. Qualitative aspects of breathlessness in health and disease. Thorax. 2009;64(8):713–718. doi: 10.1136/thx.2008.104869. [DOI] [PubMed] [Google Scholar]
- 42.Morelot-Panzini C., Gilet H., Aguilaniu B. Real-life assessment of the multidimensional nature of dyspnoea in COPD outpatients. Eur Respir J. 2016;47(6):1668–1679. doi: 10.1183/13993003.01998-2015. [DOI] [PubMed] [Google Scholar]
- 43.Lougheed M.D., Fisher T., O'Donnell D.E. Dynamic hyperinflation during bronchoconstriction in asthma: implications for symptom perception. Chest. 2006;130(4):1072–1081. doi: 10.1378/chest.130.4.1072. [DOI] [PubMed] [Google Scholar]
- 44.Banzett R.B., Pedersen S.H., Schwartzstein R.M., Lansing R.W. The affective dimension of laboratory dyspnea: air hunger is more unpleasant than work/effort. Am J Respir Crit Care Med. 2008;177(12):1384–1390. doi: 10.1164/rccm.200711-1675OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Flume P.A., Eldridge F.L., Edwards L.J., Mattison L.E. Relief of the 'air hunger' of breathholding. A role for pulmonary stretch receptors. Respir Physiol. 1996;103(3):221–232. doi: 10.1016/0034-5687(95)00094-1. [DOI] [PubMed] [Google Scholar]
- 46.Manning H.L., Shea S.A., Schwartzstein R.M., Lansing R.W., Brown R., Banzett R.B. Reduced tidal volume increases 'air hunger' at fixed PCO2 in ventilated quadriplegics. Respir Physiol. 1992;90(1):19–30. doi: 10.1016/0034-5687(92)90131-f. [DOI] [PubMed] [Google Scholar]
- 47.Banzett R.B., Lansing R.W., Brown R. Air hunger' from increased PCO2 persists after complete neuromuscular block in humans. Respir Physiol. 1990;81(1):1–17. doi: 10.1016/0034-5687(90)90065-7. [DOI] [PubMed] [Google Scholar]
- 48.Banzett R.B., Lansing R.W., Reid M.B., Adams L., Brown R. Air hunger' arising from increased PCO2 in mechanically ventilated quadriplegics. Respir Physiol. 1989;76(1):53–67. doi: 10.1016/0034-5687(89)90017-0. [DOI] [PubMed] [Google Scholar]
- 49.Petit J.M., Delhez L. [Some recent experimental data on the origin of dyspnea and ventilatory regulation in asthmatics] Acta Tuberc Pneumol Belg. 1970;61(1):169–186. [PubMed] [Google Scholar]
- 50.Binks A.P., Moosavi S.H., Banzett R.B., Schwartzstein R.M. Tightness" sensation of asthma does not arise from the work of breathing. Am J Respir Crit Care Med. 2002;165(1):78–82. doi: 10.1164/ajrccm.165.1.2105061. [DOI] [PubMed] [Google Scholar]
- 51.Moy M.L., Lantin M.L., Harver A., Schwartzstein R.M. Language of dyspnea in assessment of patients with acute asthma treated with nebulized albuterol. Am J Respir Crit Care Med. 1998;158(3):749–753. doi: 10.1164/ajrccm.158.3.9707088. [DOI] [PubMed] [Google Scholar]
- 52.Gandevia S.C., Macefield G. Projection of low-threshold afferents from human intercostal muscles to the cerebral cortex. Respir Physiol. 1989;77(2):203–214. doi: 10.1016/0034-5687(89)90007-8. [DOI] [PubMed] [Google Scholar]
- 53.Campbell E.J., Gandevia S.C., Killian K.J., Mahutte C.K., Rigg J.R. Changes in the perception of inspiratory resistive loads during partial curarization. J Physiol. 1980;309:93–100. doi: 10.1113/jphysiol.1980.sp013496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.O'Donnell C.R., Schwartzstein R.M., Lansing R.W., Guilfoyle T., Elkin D., Banzett R.B. Dyspnea affective response: comparing COPD patients with healthy volunteers and laboratory model with activities of daily living. BMC Pulm Med. 2013;13:27. doi: 10.1186/1471-2466-13-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Meek P.M., Banzett R., Parshall M.B., Gracely R.H., Schwartzstein R.M., Lansing R. Reliability and validity of the multidimensional dyspnea profile. Chest. 2012;141(6):1546–1553. doi: 10.1378/chest.11-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Harlos M. Palliative care incident pain and incident dyspnea protocol. 2014. http://palliative.info/IncidentPain.htm
- 57.Fraserhealth. Dyspnea. 2006. https://www.fraserhealth.ca/media/Dyspnea.pdf
- 58.Baker K., Barsamian J., Leone D. Routine dyspnea assessment on unit admission. Am J Nurs. 2013;113(11):42–50. doi: 10.1097/01.NAJ.0000437112.43059.a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.