Table 1. Control Studies and Ligand Optimization.
Reaction conditions: Allyl alcohol or carbonate (1.0 equiv, 0.1 mmol), DHP (1.5 equiv, 0.15 mmol), 4CzIPN (3 mol %), Ni catalyst (5 mol %), DMDC (3.0 equiv), and DMF (0.1 M) thoroughly degassed followed by stirring near blue LEDs for 16 h. Overall yields and regioselectivity were determined by GC, and E/Z isomer ratios were determined by NMR.
 | |||
|---|---|---|---|
| entry | deviation from standard conditions | yield (%) | E/Z ratio (A:B) |
| 1 | R1 = CO2Me, R2 = i-Pr, No DMDC | 83 | > 20:1 |
| 2 | none, R2 = i-Pr | 66 | > 20:1 |
| 3 | no light, R2 = i-Pr | trace | - |
| 4 | no photocatalyst, R2 = i-Pr | 0 | - |
| 5 | no Ni catalyst, R2 = i-Pr | 0 | - |
| 6 | none, R2 = Cyclohexyl | 85 | > 20:1 |
| 7 | Ni 2, R2 = Cyclohexyl | 26 | 7:1 |
| 8 | Ni 3, R2 = Cyclohexyl | 66 | > 20:1 |
| 9 | Ni 4, R2 = Cyclohexyl | 8 | E only |
| 10 | Ni 5, R2 = Cyclohexyl | 10 | 1.3:1 |
| 11 | Ni 6, R2 = Cyclohexyl | 73 | 1:1.2 |
| 12 | Ni 7, R2 = Cyclohexyl | 21 | 2:1 |
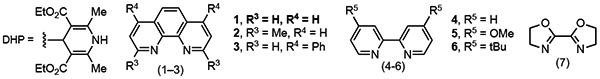 | |||
