Abstract
Synaptotagmin 1 (Syt1) is an integral membrane protein whose phospholipid-binding tandem C2 domains, C2A and C2B, act as Ca2+ sensors of neurotransmitter release. Our objective was to understand the role of individual metal-ion binding sites of these domains in the membrane association process. We used Pb2+, a structural and functional surrogate of Ca2+, to generate the protein states with well-defined protein-metal ion stoichiometry. NMR experiments revealed that binding of one divalent metal ion per C2 domain results in loss of conformational plasticity of the loop regions, potentially pre-organizing them for additional metal-ion and membrane-binding events. In C2A, a divalent metal ion in site 1 is sufficient to drive its weak association with phosphatidylserine-containing membranes, whereas in C2B, it enhances the interactions with the signaling lipid phosphatidylinositol-4,5-bisphosphate. In full-length Syt1, both Pb2+-complexed C2 domains associate with phosphatidylserine-containing membranes. Electron paramagnetic resonance experiments show that the extent of membrane insertion correlates with the occupancy of the C2 metal ion sites. Together, our results indicate that upon partial metal ion saturation of the intra-loop region, Syt1 adopts a dynamic, partially membrane-bound state. The properties of this state, such as conformationally restricted loop regions and positioning of C2 domains in close proximity to anionic lipid headgroups, “prime” Syt1 for cooperative binding of a full complement of metal ions and deeper membrane insertion.
Significance
Regulation of synaptic neurotransmission by Ca2+ requires complex protein fusion machinery. An essential part of this machinery is Synaptotagmin 1 (Syt1), a vesicular protein that triggers the membrane fusion event. Syt1 has a total of five Ca2+ sites located on its tandem C2 domains, C2A and C2B. We present evidence that the population of only one metal ion site per C2 domain results in a conformational restriction of the membrane-binding regions and is sufficient to generate a dynamic membrane-bound state of Syt1. A membrane-binding step driven by the highest-affinity Ca2+ site could be a common mechanism employed by signaling proteins that contain Ca2+-responsive C2 domains.
Introduction
The process of neurotransmitter release is tightly coupled to the changes in neuronal Ca2+ levels (1). Synaptotagmin 1 (Syt1), an integral membrane protein that is anchored to synaptic vesicles through its N-terminal region, plays a major regulatory role in this process (2, 3, 4). Together with its protein effectors, such as soluble N-ethylmaleimide-sensitive factor attachment protein receptors (SNAREs) (5,6) and complexin (7), Syt1 triggers the vesicle fusion in a Ca2+-dependent manner (8). This results in the opening of membrane fusion pore, through which neurotransmitters are released from the vesicles into the synaptic cleft (9,10).
The Ca2+-sensing function of Syt1 resides on its cytosolic, phospholipid-binding C-terminal region that comprises tandem C2A and C2B domains, connected by a 9-residue linker. These domains have five intrinsically weak Ca2+-binding sites, three on C2A and two on C2B, formed by the apical aspartate-rich loops (11,12). In the absence of anionic lipids, the population of three sites of C2A by Ca2+ occurs in a sequential, non-cooperative manner, with the high-affinity site 1 (shown in Fig. 1 A in the context of Pb2+-complexed C2 domains, vide infra) getting occupied first (13,14). In C2B, the Ca2+ affinities for two sites differ ∼2-fold, with site 1 having a higher affinity (12). Anionic phospholipids, such as phosphatidylserine (PtdSer) and phosphatidylinositol-(4,5)-bisphosphate, impart positive cooperativity on Ca2+ binding to the C2 domains (13,15). As a result, Syt1 is able to respond to Ca2+ elevation in neurons and trigger the synaptic exocytosis.
Figure 1.
High-affinity Pb2+-binding sites of Syt1. (A) Shown is a cartoon representation of the Syt1 cytoplasmic region assembled from the Pb2+-bound crystal structures of isolated C2 domains, C2A (5VFE) and C2B (5VFG). The Pb2+ affinities to site 1 exceed those of Ca2+ by ∼2 orders of magnitude. (B) Expansion of loop regions in C2A⋅Pb1 and C2B⋅Pb1 show the numbered metal ion-binding sites and the low affinity of Pb2+ to site 2. (C) Electrostatic potential maps of apo C2A and C2B are shown. (D and E) Shown are the electrostatic potential maps of loop regions with a full complement of metal ions (D) and with site 1 selectively populated by Pb2+ (E). The maps were generated using the Adaptive Poisson-Boltzmann Solver (APBS) plugin of UCSF Chimera. Protein Data Bank, PDB: 4WEE (C2A apo); PDB: 1BYN (C2A⋅Ca3); PDB: 5CCJ (C2B apo); and PDB: 1TJX (C2B⋅Ca2). To see this figure in color, go online.
Several models have been proposed to explain the membrane-binding modes of these C2 domains and their contributions to the overall Ca2+ response (16, 17, 18, 19). However, it remains unclear what the roles of individual metal ion sites are in driving the association of the C2 domains with anionic membranes. The challenge here lies in the overlapping Ca2+ affinities (11,12) and the resulting complex speciation of metal ion-bound C2 domains in solution. The role of site 1 is of particular importance as this site is predominantly populated at the initial stages of C2-membrane recruitment.
Our objective was to determine the role of the metal ion binding event to site 1 in C2-membrane interactions, which necessarily requires generating C2-metal ion complexes with well-defined stoichiometry. To overcome the challenge of overlapping Ca2+ affinities, we made use of Pb2+, a Ca2+-mimicking divalent cation (20). Heavy metal ions were shown to be useful tools to establish the C2 structure-function relationship (21, 22, 23, 24). What sets Pb2+ apart from other metal ions is two features. First, it supports the membrane interactions of C2 domains and is therefore isofunctional to Ca2+ (23,25,26). Second, although Pb2+ populates the exact same C2 domain sites as Ca2+ in solution, there are significant differences in its affinities to site 1 and 2–3: the affinity of Pb2+ to site 1 is ∼450-fold higher than that to site 2 (Fig. 1 B; (26)). These unique properties of Pb2+ with respect to its interactions with C2 domains enabled us to simultaneously achieve selective population of a specific metal-binding site, site 1, with minimal perturbation of the membrane-binding function of the proteins.
For both C2 domains of Syt1, we found that the population of site 1 by a divalent metal ion attenuates the conformational dynamics of their membrane-binding regions. Using isotropically tumbling bicelles and large unilamellar vesicles (LUVs) as membrane mimics, we characterized the membrane-binding properties of Pb2+-complexed C2 domains: individual, in tandem, and in the context of full-length Syt1 (FL-Syt1). Our work provides evidence that Syt1 can adopt a dynamic and partially membrane-bound state under conditions in which there is only a single divalent metal ion per C2 domain. Formation of such “primed” state driven by a metal ion in site 1 could be an essential step in eliciting the mutually cooperative Ca2+ and membrane-binding response.
Materials and Methods
Materials
The murine Syt1 complementary DNA was purchased from Open Biosystems (Huntsville, AL) (GE Life Sciences, Marlborough, MA). Concentrated stock solutions of Pb2+ were prepared by dissolving lead acetate tri-hydrate (Sigma-Aldrich, St. Louis, MO) in high-performance liquid chromatography (HPLC)-grade water or decalcified buffers. The necessary dilutions of this stock solution were freshly prepared before use to make working solutions. All the buffer solutions used in the experiments (MES; Sigma-Aldrich) were treated with the ion-chelating resin Chelex-100 (Sigma-Aldrich) to remove trace divalent metals before use. Lipid components used in the phospholipid vesicle as well as bicelle preparations are as follows: 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC), 1-palmitoyl-2-oleoyl-sn-glycero-3-phospho-L-serine (POPS), 1,2-dimyristoyl-sn-glycero-3-phosphocholine (DMPC), 1,2-dihexanoyl-sn-glycero-3-phosphocholine (DHPC), 1,2-dimyristoyl-sn-glycero-3-phospho-L-serine (DMPS), and L-α-phosphatidylinositol-4,5-bisphosphate (PtdIns(4,5)P2), which were obtained from Avanti Polar Lipids (Alabaster, AL). The short-chain PtdIns(4,5)P2 (di-C4-PtdIns(4,5)P2) was obtained from Echelon Biosciences (Salt Lake City, UT).
Protein expression and purification
The gene segments encoding Syt1 C2A (residues 137–265), C2B (residues 271–421), and C2AB (residues 137–421) were cloned into pET-SUMO vector (Novagen, Madison, WI) and expressed as 6×His-tagged SUMO fusion proteins in the Escherichia coli BL21(DE3) (C2A, C2AB) and Rosetta(DE3) (C2B) strains as described previously (21,26). The FL-Syt1 (residues 1–421) with the native cysteines mutated as C73A, C74A, C76A, C78A, C82S, and C277S was cloned into a pET-28a vector. For electron paramagnetic resonance (EPR) measurements, additional cysteine mutations were introduced into the cysteine-free FL-Syt1 at either positions M173C or V304C and expressed as 6×His-tagged proteins in the E. coli BL21(DE3)-RIL strain as described before (21,27). M173 and V304 are located on loop 1 of the C2A and C2B domains, respectively. We chose loop 1 because we had used it in previous studies, and it yields clear differences between aqueous and membrane-bound states (21,27). Loop 3 is also known to penetrate the bilayer, but we have no reason to think that adding more sites would change the conclusions. All mutants were prepared using the QuikChange Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA) and verified by DNA sequencing (Genewiz, South Plainfield, NJ).
Expression and purification steps from the previously described protocols were followed (21,26,27). Briefly, all SUMO fusion proteins were purified from cell lysates using HisTrap HP columns followed by the removal of 6×His-SUMO fusion tag using SUMO protease. The partially purified proteins were further refined using anion (HiTrap Q HP, C2A and C2AB) and cation (HiTrap SP HP, C2B)-exchange chromatography steps to remove any charged protein or nucleic acid impurities. The ion-exchange chromatography buffers were also supplemented with 100 μM EDTA to ensure that the purified Syt1 domains are free of metal contamination. Immediately before use, the protein stock solutions were concentrated and subjected to four successive passes through the desalting PD MidiTrap G-25 columns to attain complete buffer exchange and removal of EDTA.
For the NMR measurements, individual C2 domains were uniformly enriched with 15N, as described previously (21,26). The C2AB fragment was additionally deuterated to a level of ∼80% by growing E. coli on the M9 medium supplemented with D2O and BioExpress 1000 (10 mL/L; [U-2H 98%, U-15N 98%]). The isotopically enriched chemicals were purchased from Cambridge Isotope Laboratories (Tewksbury, MA). Unless specified otherwise, all isotopically enriched protein preparations were exchanged post-purification into an NMR buffer of the following composition: 20 mM MES (pH 6.0), 100 mM KCl, 8% D2O, and 0.02% NaN3.
FL-Syt1 used for the EPR experiments was purified in 3-[(3-cholamidopropyl)dimethylammonio]-1 propanesulfonate (CHAPS) by affinity chromatography using Ni-NTA Agarose Resin (QIAGEN, Hilden, Germany). The protein was spin labeled overnight at 4°C using the thiol-specific spin label 1-oxy-2,2,5,5-tetramethylpyrrolinyl-3-methyl methanethiosulfonate (MTSL) while bound to the Ni-NTA column. The labeled protein was eluted followed by removal of the 6×His tag by thrombin cleavage and further purified by cation-exchange chromatography (HiTrap SP HP).
Preparation of phospholipid vesicles and bicelles
LUVs of the desired compositions were prepared by aliquoting the chloroform solutions of the POPC/POPS lipids, followed by extensive vacuum drying and extrusion in NMR buffer (Mini-Extruder; Avanti Polar Lipids). All LUV preparations were verified for the mean diameter of 100 nm by dynamic light scattering and used within 2 days of preparation. Phospholipid concentrations in LUV solutions were calibrated using the phosphate determination assay (28).
To prepare isotropically tumbling bicelles, chloroform solutions of DMPC and DHPC were aliquoted, extensively dried under vacuum, and resuspended in NMR buffer. DMPC preparation was vortexed and subjected to three rapid freeze-thaw cycles to create a homogeneous slurry. Clear DHPC solution was then added to achieve two-fold molar excess to DMPC (q = 0.5). The resulting mixture was briefly vortexed and subjected to four rapid freeze-thaw cycles, resulting in clear homogeneous bicelle stock solutions. Total lipid concentration was verified using phosphate determination assay (28). Additional long-chain lipid components, such as DMPS and PtdIns(4,5)P2, were dried and added during the bicelle preparation wherever applicable. The bicelle stock solutions were flash frozen and stored at −20°C. Before use, the frozen stocks were thawed at 42°C, followed by incubation at room temperature.
NMR spectroscopy
All NMR experiments were conducted at 298.2 K, unless specified otherwise; the temperature was calibrated using deuterated methanol. NMR data were processed with NMRPipe (29) and analyzed with Sparky (30). Curvefit program (Arthur G. Palmer’s laboratory, Columbia University, New York, NY) was used to fit the relaxation data and extract relaxation parameters. The NMR observables, such as relaxation rate constants and chemical shift perturbations, were measured for all spectrally resolved N-H groups and plotted against the primary structure.
Measurements of 15N transverse relaxation rate constants, R2
The R2 values were measured on an NMR spectrometer (Avance III HD; Bruker Biospin, Billerica, MA) operating at the 1H Larmor frequency of 600 MHz (14.1 T) and equipped with a cryogenically cooled probe. NMR samples contained 300 μM [U-15N]-enriched C2A or C2B, each in the absence or presence of stoichiometric amounts of Pb2+. Additional relaxation measurements were conducted on the Pb2+-complexed C2A domain in the presence of LUVs. The site-specific R2,CPMG values (31) were extracted from fitting the 15N-1HN crosspeak intensity decays at seven unique and three duplicate relaxation time points ranging from 8 to 140 ms. The free-precession R2,HE values (32) were measured to estimate the exchange contribution to the observed relaxation behavior and the timescale of exchange, both through direct comparison with the R2,CPMG values. The Hahn-Echo delay was set to 48.2 ms. All relaxation data were acquired in the interleaved manner.
NMR-detected interactions between Syt1 domains and their ligands
C2AB and Pb2+
Binding of Pb2+ to C2AB was monitored using 15N-1H transverse relaxation-optimized spectroscopy (TROSY)-heteronuclear single quantum coherence (HSQC) spectra collected on an NMR spectrometer (Avance III HD; Bruker Biospin) operating at the 1H Larmor frequency of 800 MHz (18.8 T) and equipped with a cryogenically cooled probe. Pb2+ was added to the NMR sample containing 100 μM [U-15N, ∼80%-2H] C2AB at one and two-fold stoichiometric excess. The chemical shift perturbations (CSPs, Δ) due to Pb2+ interactions with proteins were calculated using the following equation:
| (1) |
where ΔδH and ΔδN are residue-specific 1H and 15N chemical shift differences between metal-free and Pb2+-complexed states of C2AB.
Syt1 domains and bicelles
Binding of isotropic anionic bicelles to metal-free and Pb2+-complexed C2A, C2B, and C2AB was conducted at 18.8 T and 300 K. A series of 15N-1H HSQC (TROSY-HSQC) spectra were acquired at each sample condition for the C2A/C2B (C2AB) domains. Protein concentration was kept at 150 μM. The bicelles were added to a total lipid concentration of 60 mM. The bilayer compositions of the added bicelles were either DMPC:DMPS:PtdIns(4,5)P2 = 84:14:2 or DMPC:DMPS = 85:15. The chemical shift perturbations induced by bicelle interactions were calculated using Eq. 1. The absolute resonance intensities for the bicelle-containing samples were normalized to their respective bicelle-free reference counterparts to estimate the signal attenuation.
C2A and LUVs
The interaction of the C2A domain with LUVs composed of either pure POPC or POPC:POPS = 80:20 was monitored at 14.1 T using a 100 μM sample of [U-15N] C2A. A series of 15N-1H HSQC spectra were collected in the presence of LUVs, with the total lipid concentration ranging from 0 to 2.0 mM. Residue-specific intensity changes were distributed into linearly and exponentially decaying patterns based on their behavior, and the respective fitting models were used to extract the “decay” rates.
C2B and di-C4-PtdIns(4,5)P2
Interactions of C2B with di-C4-PtdIns(4,5)P2 were monitored at 14.1 T, using 100 μM C2B in either apo- or Pb2+-complexed forms. The di-C4-PtdIns(4,5)P2 concentrations were: 0, 10, 50, 100, 150, 300, and 550 μM for the C2B·Pb1 sample and 55, 100, 150, 250, 400, 600, 800, and 1000 μM for the apo C2B sample. Residue-specific chemical shift perturbation values Δ were calculated using Eq. 1. The binding curves were constructed by plotting Δ as a function of di-C4-PtdIns(4,5)P2 concentration. To extract the dissociation constant Kd, the curves were globally fitted (C2B·Pb1: seven residues; apo C2B: five residues) with the single-site binding model:
| (2) |
where Δmax is the maximal perturbation, and P0 and L0 are the total protein and di-C4-PtdIns(4,5)P2 concentrations, respectively.
Measurements of EPR spectra
For EPR measurements, FL-Syt1 was reconstituted into POPC:POPS = 85:15 at a 1:200 protein to lipid ratio and dialyzed into metal-free buffer (20 mM HEPES and 150 mM KCl (pH 7.4)) in the presence of Bio-Beads (Bio-Rad Laboratories, Hercules, CA) (27). Pb2+ was then added to samples at either a 2:1 Pb2+:FL-Syt1 ratio or in 12- to 50-fold excess. Measurements were taken as described previously, using a Bruker X-Band EMX spectrometer (Bruker Biospin) equipped with an ER 4123D dielectric resonator. Continuous wave spectra were taken with 100 G magnetic field sweep, 1 G modulation, and 2.0-mW incident microwave power at room temperature. 10 μL samples of protein with concentrations varying between 15 and 75 μM, depending on the desired Pb2+:FL-Syt1 ratio, were prepared in glass capillary tubes (0.60 mm inner diameter × 0.84 mm outer diameter round capillary; VitroCom, Mountain Lakes, NJ). The phasing, normalization, and subtraction of EPR spectra were performed using in-Lab software written by David Nyenhuis and LabVIEW software provided by Dr. Christian Altenbach (27). Progressive power saturation of the EPR spectrum was used to determine nitroxide membrane depth and was performed as previously described (21,27). Samples were run in gas-permeable TPX-2 capillaries, and the values of ΔP1/2 obtained in air and in the presence of Ni(II)EDDA were used to calculate a depth parameter, Φ (33). The spin label depth was then estimated using the empirical expression:
| (3) |
where x is the distance of the spin label from the lipid phosphate plane (positive x values are inside, whereas the negative values are outside the bilayer), and A, B, C, and D are empirically determined constants (21,27).
Results
Electrostatic properties of C2 domains in different states of metal ligation
We previously obtained high-resolution crystal structures of individual Pb2+-complexed Syt1 C2 domains, in which Pb2+ ions bind to site 1 with two-orders of magnitude higher affinity than Ca2+ (Fig. 1, A and B; (26)). To determine how metal ion binding to site 1 alters the electrostatic profiles of these C2 domains, we calculated the surface electrostatic potential maps of the proteins in different states of metal ligation. The negatively charged metal ion-binding region is formed by the aspartate-rich loops (Fig. 1 C). In the presence of a full complement of Ca2+ ions, C2A undergoes a prominent electrostatic shift in which the intra-loop potential changes from negative to positive (Fig. 1 D). The effect of bound Ca2+ on the same region in C2B is the neutralization of the negative charge (Fig. 1 D). This is because C2A can accommodate three Ca2+ ions within the intra-loop region as compared with the C2B’s maximal of two. Pb2+ binding to site 1 partially neutralizes the intra-loop region in both domains. Because of the differences in the arrangement of basic residues, loop 2 of C2A becomes more electropositive, whereas loop 2 of C2B becomes neutral (Fig. 1 E). The differences in electrostatic properties between the two C2 domains determine how the single metal ion-bound states interact with anionic membranes (vide infra).
Population of site 1 by a metal ion alters the conformational plasticity of the membrane-binding regions
In addition to electrostatics, the conformation and dynamics of the membrane-binding regions in the C2⋅Pb1 complexes can have profound influence on the membrane association. The average conformation of the domains, as it exists in the crystalline state, changes only moderately upon metal ion binding. The ms-to-μs, but not the sub-nanosecond, backbone dynamics in a related C2 domain were previously shown to be responsive to the state of metal ligation (34). We therefore investigated the ms-to-μs dynamics of the Syt1 C2 domains in apo and single Pb2+ states using solution NMR methods.
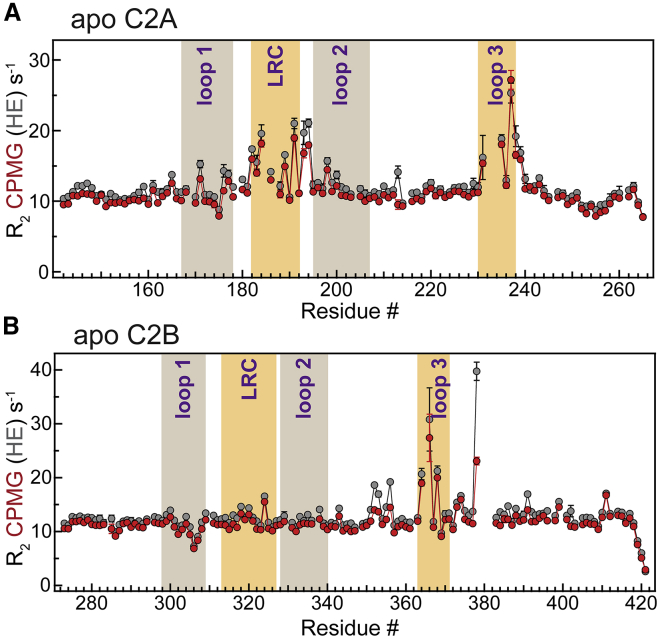
Two types of NMR parameters, R2,HE and R2,CPMG, were measured for all spectrally resolved N-H groups of the C2 domain backbone. R2,HE is a free-precession transverse relaxation rate constant whose elevated values are indicative of ms-to-μs conformational exchange processes. R2,CPMG is a transverse relaxation rate constant whose elevated values reflect dynamics on timescales faster than 100 μs. A comparison of the two transverse relaxation rate constants enables one to estimate, in a residue-specific manner, the timescale of motions present in macromolecules.
The NMR relaxation data for the apo C2 domains are presented in Fig. 2. Based on the elevated R2,HE values (Fig. 2 A), the backbone of apo C2A has two highly dynamic regions: loop 3, which participates in both metal ion coordination and binding to PtdSer-rich membranes, and the lysine-rich cluster (LRC). Surprisingly, there is little dynamics in loop 1, which provides roughly half of the oxygen ligands when site 1 gets populated by a divalent metal ion.
Figure 2.
The membrane-binding regions of apo C2A and C2B are dynamic on the microsecond timescale. R2,HE (gray) and R2,CPMG (red) are plotted against the amino acid sequence of C2A (A) and C2B (B). The regions corresponding to metal ion coordinating loops and LRCs are highlighted. To see this figure in color, go online.
The dynamics profile is different for C2B (Fig. 2 B). The most significant difference between C2B and C2A is located in the LRC and shows no conformational exchange in C2B but is highly dynamic in C2A. The functional role of LRC in C2B domains is to provide an additional docking site for the negatively charged signaling lipids, such as PtdIns(4,5)P2, and thereby increase the residence time at the membrane (35). Another noteworthy difference is that although in both domains, loop 3 undergoes conformational exchange, in only C2B, the regions adjacent to loop 3 hinges are also dynamic. To evaluate the timescale, we inspected the difference plots between the R2,HE and R2,CPMG values. In both domains, the attenuation of dynamics due to the application of the Carr-Purcell-Meiboom-Gill (CPMG) pulse train is modest (Fig. S1, A and B). These data indicate that the timescale of loop 3 and LRC (in C2A) motions is faster than 100 μs.
To determine how the metal ion binding to site 1 influences the backbone dynamics, we prepared single Pb2+ complexes of the C2 domains and measured their R2,HE and R2,CPMG values. Under 1:1 stoichiometric conditions of our sample, site 1 is slightly under-populated. Although the population of apo proteins is very low (∼2–3%), the inter-conversion between apo and metal ion-bound species can lead to large chemical exchange effects on the millisecond timescale, as was previously shown for the Ca2+ ion binding to the C2A variant (36). Indeed, we observed a significant elevation of the R2,HE values in the loop regions of both domains. Fortunately, these processes occur on a timescale of ms and therefore can be efficiently suppressed by an application of the CPMG pulse train (Fig. S2). Therefore, the R2,CPMG values in the C2·Pb1 complexes report almost exclusively on the intrinsic dynamics of the loops.
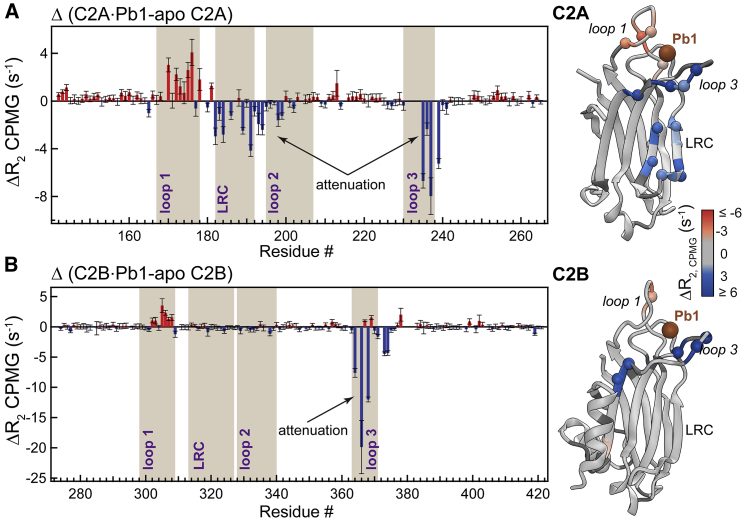
The R2,CPMG differences between the Pb2+-complexed and apo proteins revealed that metal ion binding to site 1 attenuates the dynamics of loop 3 (Fig. 3). This is manifested in large negative values of ΔR2,CPMG in loop 3 for both domains. In addition, the entire positively charged LRC region of C2A loses its conformational plasticity (Fig. 3 A). This indicates that the dynamic effect of metal ion binding to site 1 propagates as far as ∼15–25 Å throughout the C2A domain in what could be an allosteric communication between the metal ion binding sites and LRC. Loop 1, which is immobile on the ms-to-μs timescales in the apo states of C2A and C2B (Fig. 2), experiences a slight increase in R2,CPMG because of incomplete suppression of the ms timescale chemical exchange due to metal ion binding (vide supra).
Figure 3.
Binding of a single metal ion to C2 attenuates the μs-timescale dynamics of the membrane-binding regions. Difference plots of the R2,CPMG values measured in apo and Pb2+-complexed C2 domains are shown for the C2A (A) and C2B (B) domains. Negative values indicate the attenuation of dynamics. The R2,CPMG differences are color coded and mapped onto the 3D structures of the Pb2+-complexed C2 domains. To see this figure in color, go online.
For the function of C2 domains, the implications of the attenuated dynamics are two-fold. First, conformational restrictions brought about by a metal ion in site 1 may facilitate membrane interactions through imposing loop 3 orientation that is favorable for membrane insertion. Second, the geometric restriction of the Asp ligands, some of which are shared by metal ions in sites 1 and 2, will likely facilitate the subsequent metal ion binding to site 2. In either case, the effect of populating site 1 with a metal ion would “prime” the C2 domains of Syt1 for metal ion-dependent membrane interactions.
C2A weakly associates with anionic membranes in a single metal ion-bound state
We then asked if the population of site 1 by a metal ion is sufficient to support the membrane interactions of the Syt1 C2 domains. Starting with C2A, we prepared the [U-15N]-enriched C2A·Pb1 complex and mixed it with the preparation of isotropically tumbling bicelles that mimic the membrane environment. Two anionic phospholipids, PtdSer and PtdIns(4,5)P2, were incorporated into the bicelles at 14 and 2%, respectively, relative to the long-chain phospholipid. The low fraction of PtdIns(4,5)P2 brings about the question of its distribution in the bicelles. This distribution is stochastic, and some heterogeneity in the bicelle population with respect to PtdIns(4,5)P2 content is expected. All NMR observables measured in bicelles represent the average over this bicelle ensemble.
Comparison of the [15N-1H] HSQC spectra with and without bicelles (Fig. 4 A) revealed two spectroscopic signatures: chemical shift perturbations (CSPs, Δ) of the N-H cross-peaks and attenuation of their intensities. The CSPs affected loop regions 1, 2, and 3 of the C2A domain but not the LRC (Fig. 4, B and D). No chemical shift perturbations were observed when C2A domain in the absence of any metal ions was mixed with bicelles, indicating that CSPs report specifically on the metal ion-dependent interactions of the C2A domain with membranes. Lack of CSPs in the LRC indicates that this region does not directly interact with PtdSer and PtdIns(4,5)P2. In the membrane-free system, a similar interaction pattern was observed between the Ca2+-complexed C2A domain and inositol-1,4,5-triphosphate (35).
Figure 4.
Syt1 C2A·Pb1 complex interacts with anionic bicelles. (A) Shown is an overlay of the [15N-1H] HSQC spectral regions of C2A·Pb1 in the absence (green) and presence (purple) of anionic bicelles that illustrates CSPs experienced and broadening of protein resonances upon interactions with bicelles. (B) CSP plot showing that perturbations are localized primarily to the loop regions. (C) Attenuation of peak intensities in the C2A·Pb1 complex upon the addition of anionic bicelles, expressed as residue-specific I/I0 ratios, in which I and I0 are the peak intensities in the presence and absence of bicelles, respectively. Loop regions are preferentially broadened because of chemical exchange. (D) CSP values were mapped on the C2A·Pb1 complex (PDB ID: 5VFE) illustrating the proximity of the affected regions to the metal ion-binding site 1. (E) Comparison of the residue-specific I/I0 ratios in apo C2A and C2A·Pb1 is shown. The overall intensity attenuation is significant in the C2A·Pb1 sample but negligible in the apo C2A sample, in which it merely reflects an increase in solution viscosity due to bicelle addition. (F) Shown is a schematic representation of the C2A·Pb1-bicelle complex that is consistent with our experimental data. To see this figure in color, go online.
The intensity attenuation of the C2A·Pb1 N-H cross-peaks, expressed as the ratio of intensities in the bicelle-containing (I) and bicelle-free (I0) samples, varied across the primary structure (Fig. 4 C). The regions with the most reduced intensities correspond to the protein loops and mirror the CSP pattern. This is a manifestation of a chemical exchange process whose kinetics is in the intermediate-to-fast regime on the NMR chemical shift timescale. It is well established that the PtdSer can coordinate the C2-bound metal ions while interacting simultaneously with the loop residues (37,38). We conclude that the chemical exchange process represents the binding of the binary C2A·Pb1 complex to the bicelles, with the formation of the ternary C2A·Pb1·PtdSer (bicelle) complex. This conclusion is further reinforced by the comparison of the residue-specific I/I0 ratios between apo C2A and C2A·Pb1 in the presence of bicelles (Fig. 4 E). The intensity ratios are systematically lower for the C2A·Pb1 complex and not just for the loop regions. The overall decrease in intensities is due to the increase in the apparent rotational correlation time of C2A·Pb1 because it partially associates with bicelles that are slow-tumbling high-molecular-mass entities. The population of the membrane-bound C2A·Pb1 complex is low, based on the relatively modest attenuation of cross-peak intensities corresponding to regions not subject to chemical exchange.
The C2A·Pb1 association with membranes also occurs in the absence of PtdIns(4,5)P2, in which the only anionic lipid component is PtdSer. We demonstrated this by conducting NMR-detected experiments of the C2A·Pb1 binding to LUVs (Fig. S3). Consistent with our bicelle data, the population of the membrane-bound state of C2A·Pb1 is low, evidenced by the moderate systematic attenuation of cross-peak intensities (Fig. S4, purple bars). Adding more Pb2+ to solution results in the population of site 2 of the C2A domain. This increases the fraction of membrane-bound Pb2+-complexed C2A as manifested in the ∼2-fold decrease in the N-H cross-peak intensities (Fig. S4, green bars).
Taken together, our data indicate that population of site 1 by a divalent metal ion is sufficient to drive weak association of the C2A domain with anionic membranes. The membrane-interacting regions primarily involve the metal-binding loops, as shown schematically for the C2·Pb1·bicelle complex in Fig. 4 F.
C2B interactions with anionic membranes are enhanced by metal ion binding to site 1
The C2B domain in Syt1 is distinct from C2A with respect to its electrostatic properties. The unique electrostatic makeup of the C2B surface provides an additional complexity and control to the membrane interactions of Syt1 (12,19,39,40). The LRC of C2B has six basic residues, whereas that of C2A has four basic residues that are flanked by two acidic ones. This makes the LRC of C2B significantly more electropositive (see Fig. 1 C), turning it into an effective PtdIns(4,5)P2 sensor (41). Moreover, there are additional basic residues, Arg 398 and Arg 399, at the C2B end opposite to the loop region. These residues are implicated in the membrane-apposition process (17,40). In the presence of Ca2+, the basic regions can create scaffolds by simultaneously interacting with various lipid and protein partners such as PtdSer, inositol polyphosphates, Ca2+ channels, and SNAREs (15,19,42,43). In the absence of Ca2+, the LRC of C2B is believed to pre-associate with the PtdIns(4,5)P2-rich plasma membranes (44).
Using the same experimental strategy as for C2A, we prepared the (U-15N)-enriched C2B·Pb1 and collected [15N-1H] HSQC spectra in the presence and absence of anionic bicelles (Fig. 5 A). Extensive line broadening accompanied by significant chemical shift changes was observed for many N-H crosspeaks of C2B. The CSP pattern calculated for the bicelle-containing samples relative to the bicelle-free C2B·Pb1 was clearly distinct from what we observed for the C2A·Pb1 complex in two aspects: 1) the CSPs for loop 3 of C2B were significantly larger than those for loop 1, suggesting a more prominent role of loop 3 in protein-membrane interactions; and 2) there were very significant CSPs in the LRC loop 2 region (compare Figs. 4 B and 5 B). Mapping the CSP values onto the three-dimensional (3D) structure of the Pb2+-complexed C2B domain highlighted a contiguous surface likely to be involved in interactions with anionic membranes (Fig. 5 D). Another drastic difference between C2A·Pb1 and C2B·Pb1 was in the extent of signal attenuation upon the addition of bicelles (compare Figs. 4 C and 5 C). The intensity drop for the C2B·Pb1 was uniform, with the exception of a few flexible C-terminal residues, and substantial: the mean I/I0 value from the 5% trimmed data set was 0.14. We conclude that the C2B·Pb1 complex, in contrast to C2A·Pb1, associates with anionic bicelles with a high affinity.
Figure 5.
C2B·Pb1 binds to anionic bicelles with high affinity. (A) Shown is an overlay of the [15N-1H] HSQC spectral regions of C2B·Pb1 in the absence (red) and presence (blue) of anionic bicelles that illustrates CSPs and a broadening of protein resonances upon interactions with bicelles. (B) CSP plot shows that perturbations are localized primarily to the LRC and loop 3 of the C2B domain. (C) Shown is an attenuation of peak intensities in the C2B·Pb1 complex upon the addition of anionic bicelles, expressed as residue-specific I/I0 ratios, in which I and I0 are the peak intensities in the presence and absence of bicelles, respectively. Significant uniform (with the exception of the flexible C-term residues) attenuation indicates high-affinity association of C2B·Pb1 with bicelles. (D) CSP values mapped on the C2B·Pb1 complex (PDB ID: 5VFG) cover a contiguous surface that includes primarily loop 3 and the LRC. (E) Correlation of residue-specific I/I0 ratios in apo C2B and C2B·Pb1 in the presence of PtdIns(4,5)P2 (red) indicates that metal ion binding to site 1 contributes moderately to protein-bicelle interactions. Omitting PtdIns(4,5)P2 abolishes the interactions of C2B·Pb1 with bicelles (green). (F) Shown is a schematic representation of the C2B·Pb1-bicelle complex that is consistent with our experimental data. To see this figure in color, go online.
To establish the metal ion dependence of this process, we conducted a control experiment in which the metal ion-free C2B domain (apo C2B) was mixed with anionic bicelles of the same composition. The observed CSP values were smaller than those for the C2B·Pb1 complex, especially for loop 3 (Fig. S5). The correlation of I/I0 intensity ratios between apo C2B and C2B·Pb1 showed systematically lower values for the latter, suggesting that the fractional population of the membrane-bound C2B·Pb1 species is higher than that of the apo species (Fig. 5 E, red symbols). However, the overall effect is rather modest, suggesting that the presence of the metal ion at site 1 contributes moderately to the C2B-membrane interactions. The binding of C2B·Pb1 to bicelles is significantly enhanced by PtdIns(4,5)P2; when we omitted PtdIns(4,5)P2, the I/I0 ratios increased to values of 0.8 and above (Fig. 5 E, green symbols).
Our data are consistent with the orientation of C2B·Pb1 in which the LRC and loop 3, but not loop 1, are in close membrane contact (Fig. 5 F). In the previously reported model of the C2B-membrane complex under the saturating Ca2+ conditions (18,19), C2B has both loops 1 and 3 inserted into the membrane, whereas its LRC makes contact with PtdIns(4,5)P2. The implication is that the metal ion binding to site 2 likely causes the insertion of loop 1 into the headgroup region and “tilts” the C2B by reducing the angle between its long axis and the bilayer normal.
The affinity of C2B to PtdIns(4,5)P2 is enhanced by metal ion at site 1
Binding of a full complement of Ca2+ ions enhances the affinity of C2 domains to PtdIns(4,5)P2 (25,45). To evaluate the thermodynamic gain afforded exclusively by site 1, we conducted NMR-detected binding experiments between C2B (apo and the C2B·Pb1 complex) and di-C4-PtdIns(4,5)P2. The rationale behind using water-soluble di-C4-PtdIns(4,5)P2, as compared with inositol 1,4,5-trisphosphate, is that it has the same electrostatic properties of the headgroup as the longer-chain PtdIns(4,5)P2.
Both C2B species responded to an increasing concentration of PtdIns(4,5)P2 in a titratable manner (Figs. 6 A and B). The binding process is in the fast exchange regime relative to the NMR chemical shift timescale. This is manifested in a smooth trajectory of the N-H cross-peaks as a function of an increasing concentration of ligand in both apo C2B and C2B·Pb1 [15N-1H] HSQC spectra. The fast exchange behavior enabled us to construct the binding curves that represent the chemical shift changes experienced by the N-H crosspeaks, Δ, as a function of the total concentration of di-C4-PtdIns(4,5)P2. Binding curves for well-resolved residues that showed a combined Δ of >0.05 ppm were globally fitted to obtain the following dissociation constants: Kd = 102 ± 3 μM (C2B·Pb1) and 215 ± 16 μM (apo C2B). We conclude that the presence of metal ion in site 1 of the C2B domain alone is sufficient to cause a two-fold increase of affinity to PtdIns(4,5)P2. A comparison of the CSP patterns (Fig. S6) mapped onto the protein 3D structures (Fig. 6 C) shows that the same regions in apo C2B and the C2B·Pb1 are influenced by the PtdIns(4,5)P2 interactions: the LRC and loop 3-adjacent β7 segment (Fig. 6 B). These regions form a concave site that is typical for C2 domains with specificity toward phosphoinositides (41,46).
Figure 6.
Binding of Pb2+ at site 1 enhances the affinity of C2B toward PtdIns(4,5)P2. (A and B) Overlays of the [15N-1H] HSQC spectral regions show progressive chemical shift changes upon the addition of di-C4-PtdIns(4,5)P2 to the C2B·Pb1 complex (A) and apo C2B (B). N-H crosspeaks that illustrate the binding of di-C4-PtdIns(4,5)P2 to the canonical site in the LRC are labeled. Insets: NMR-detected binding curves for residues that either form or are in close proximity to the canonical binding site are shown. The effective Kd values extracted from the global fit of the curves are 102 μM (C2B·Pb1) and 215 μM (apo C2B). (C) Chemical shift perturbations Δ due to di-C4-PtdIns(4,5)P2 binding (0–0.4 mM range) were mapped onto the crystal structures, the C2B·Pb1 complex (5VFG) and apo C2B (5CCJ). Positively charged residues that form LRC are shown in stick representation. Additionally, residues experiencing Δ larger than 0.15 ppm are labeled. To see this figure in color, go online.
The two-fold increase in affinity represents the thermodynamic enhancement that is attributed solely to the interactions of the LRC residues with PtdIns(4,5)P2. In neuronal membranes, an additional favorable contribution to the protein-membrane interactions would come from higher-abundance anionic phospholipids, such as PtdSer, which directly coordinates the metal ion bound to site 1 of C2B (37).
The membrane interaction pattern of C2AB is similar to that of individual domains
The tandem C2A and C2B domains (Fig. 1 A) constitute the membrane-binding unit of Syt1 (12,47). Their distinct electrostatic properties and preferences toward binding partners govern the Syt1-mediated vesicle fusion (5,19,27). The question of the C2AB conformational preferences has elicited some discussion in the literature. In the crystalline state, C2AB can adopt a “closed” conformation with well-defined inter-domain interface (48). In solution, the atomic force microscopy and intramolecular Förster resonance energy transfer measurements by Chapman and co-workers indicate that there exists an interaction between C2A and C2B (49) and that it is important for the Syt1 function (50). However, NMR experiments conducted by Rizo’s group argue against the presence of a well-defined inter-domain interface in solution (17). What is evident is that there exists significant inter-domain conformational flexibility in the C2AB fragment. We sought to determine how this flexibility influences membrane interaction properties under limiting metal ion conditions.
Using the C2AB fragment, we first tested if Pb2+ populates the same metal ion binding sites as it does in the isolated domains. Because of the acidic nature of the 9-residue linker (there are four glutamate residues, Fig. 1 A), there was a possibility that this region could sequester added Pb2+. [15N-1H] TROSY-HSQC spectra of [U-15N, ∼80%-2H] C2AB at 1:1 and 1:2 protein to Pb2+ ratios (Fig. S7) revealed the same chemical exchange regime, saturation behavior, and chemical shift perturbation patterns as for the isolated C2 domains (26). These data indicate that Pb2+ selectively populates site 1 of the C2A and C2B domains in the context of the C2AB fragment and that the linker region is not involved in metal ion binding. The state of C2AB with Pb2+ bound to site 1 in both domains is subsequently referred to as C2AB·Pb1.
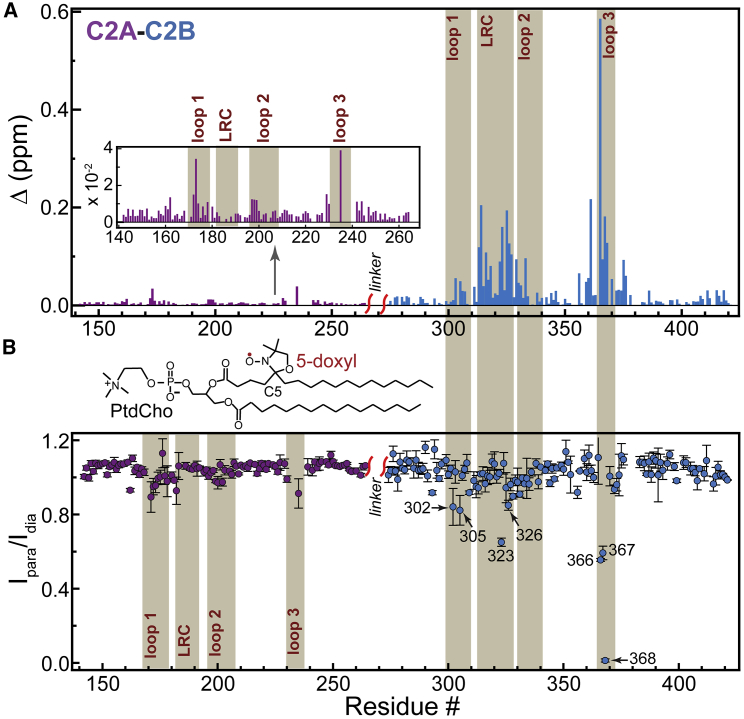
The addition of anionic bicelles to the C2AB·Pb1 complex produced the following spectroscopic signatures in the [15N-1H] TROSY-HSQC spectra: decrease of the N-H cross-peak intensities and chemical shift perturbations, both of which are indicative of the protein-membrane-binding process. The percentage of intensity decrease was rather uniform within each domain, but the extent was different: 28 and 55% on average for the C2B and C2A domains, respectively. The pattern of chemical shift perturbations and their magnitude, small for the C2A domain loops and large for the LRC and loop 3 of the C2B domain (Fig. 7 A), mirrored the data obtained for the individual domains (Figs. 4 B and 5 B).
Figure 7.
The C2AB·Pb1 complex binds to anionic bicelles with both domains contacting the membrane. (A) Loop 3 and LRC of the C2B domain make a dominant contribution to the chemical shift perturbation experienced by the C2AB·Pb1 complex upon binding anionic bicelles. The C2A data are shown in the inset with the rescaled y axis. The CSP patterns of the C2 domains in the context of the C2AB fragment-bicelle system are similar to those of the individual domains shown in Figs. 4B and 5B. The similarities refer to both the overall pattern and the magnitude. (B) Membrane contact of C2A and C2B is revealed through the attenuation of the C2AB·Pb1 peak intensities upon the addition of anionic bicelles containing a paramagnetic lipid, 5-doxyl PtdCho, at ∼3 molecules per leaflet. The attenuation is expressed as residue-specific Ipara/Idia ratios, in which Ipara and Idia are the peak intensities in the presence and absence of paramagnetic lipids, respectively. To see this figure in color, go online.
We used the paramagnetic relaxation enhancement (PRE) of N-H groups to evaluate the extent of membrane contacts of C2 domains in the C2AB fragment. A lipid bearing a paramagnetic doxyl tag, 5-doxyl phosphatidylcholine (PC), was introduced into the bicelles to give ∼3 molecules per leaflet (Fig. 7 B). The unpaired electron of the doxyl group enhances the transverse relaxation rates of neighboring spins, resulting in the broadening of the corresponding N-H cross-peaks and a reduction in their intensities. The label position is such that N-H groups penetrating the headgroup region would experience the most broadening. If the protein-membrane-binding process is in fast exchange on the NMR chemical shift timescale, which is the most likely scenario for the C2AB·Pb1 complex, the PRE effect is scaled by the population of paramagnetic species.
The residue-specific PRE values were calculated as the ratios of protein peak intensities in the C2AB·Pb1 samples containing diamagnetic and paramagnetic preparations of bicelles, Idia/Ipara (Fig. 7 B). Consistent with the chemical shift perturbation data of Fig. 7 A, the C2A domain showed a weak but readily discernable PRE effect in the loop regions. The PRE values were significantly larger for the C2B domain, with the residues of loop 3 (K366, I367, and G368) and LRC (L323 and K326) showing >15% intensity attenuation. Taken together, our data on the C2AB·Pb1 complex indicate that both domains make membrane contact when site 1 on each domain is populated by a divalent metal ion. These results support the existence of a “primed” membrane-associated state under limiting metal ion conditions. In such a state, PtdIns(4,5)P2-mediated membrane docking of C2B will be augmented by the metal ion at site 1 and keep both domains anchored at the membrane interface. The membrane binding of C2A will remain weak. This will ensure a strict metal ion-concentration dependence of the membrane insertion process for the C2A that requires a full complement of metal ions to associate with PtdSer-containing membranes.
Progressive saturation of metal ion binding sites in FL-Syt1 correlates with the extent of membrane penetration
To determine how the occupancy of metal ion binding sites influences the membrane insertion of the C2 domains in FL-Syt1, we conducted EPR experiments on the protein reconstituted into anionic LUVs. Under the conditions of our EPR experiments, both C2 domains of Syt1 bind in cis with respect to the transmembrane segment that anchors the protein to the LUVs (27). The spin-labeled side-chain R1 (Fig. 8) was attached to either loop 1 site in C2A (173R1) or a loop 1 site in C2B (304R1). Both sites are known to penetrate negatively charged membranes under saturating Ca2+ conditions. When no metal ions are present in solution, the R1 side chain is in the aqueous phase and hence highly mobile, producing narrow EPR line shapes for both C2 domains (Fig. 8, black traces). The addition of two-fold molar Pb2+ excess with respect to protein selectively populates site 1 in C2A and C2B. The EPR spectra under these conditions show significant broadening indicative of motional restriction due to partitioning of R1 into membranes (red trace in Fig. 8). There are two components: the broad component resembles that from a membrane-bound state of the C2 domains, and the more mobile component resembles that from an aqueous state. In these spectra, the membrane-bound state dominates and represents 85% or more of the total protein. The addition of Pb2+ at a molar ratio (Pb2+:Syt1) of 20:1 eliminates the small aqueous population, further broadening the spectra and giving rise to a fully bound state (blue trace in Fig. 8). This occurs for both domains and suggests that with only partial saturation of the domains with Pb2+, there is equilibrium between membrane inserted and aqueous populations that is eliminated with excess metal.
Figure 8.
Membrane insertion of C2 domains in FL-Syt1 depends on the occupancy of metal ion binding sites. Shown are x-band EPR spectra of full-length membrane-reconstituted Syt1, in which the first Ca2+ binding loop of either the C2A domain (site 173) or the C2B domain (site 304) was labeled with 1-oxy-2,2,5,5-tetramethylpyrrolinyl-3-methyl methanethiosulfonate to produce the R1 side chain. Pb2+ binding to Syt1 promotes membrane insertion of the domains that are dependent upon the occupancy of the sites in the binding loops. The additional broadening in the EPR spectra with excess Pb2+ (Pb2+:Syt1, 20:1) suggests a deeper average membrane penetration of the domains in this state. The ratio of Pb2+:Syt1 was varied from 12:1 to 50:1 with no change in the EPR spectra. The EPR spectra in the state labeled “No metal” were obtained after the addition of 4 mM EGTA, and these spectra were identical to spectra obtained before the addition of Pb2+. To see this figure in color, go online.
We used progressive power saturation of the EPR spectra and a collision-gradient approach to determine the membrane depth of 173R1 and 304R1. Table 1 gives the depth parameters and membrane depths under two Pb2+ concentration conditions. When site 1 is occupied by a metal ion, both 173R1 (C2A) and 304R1 (C2B) have apparent positions on the aqueous side of the phosphate groups, with average distances of 0.8 and 3.5 Å, respectively, above the level of lipid phosphates. These depths likely reflect contributions from both the membrane inserted and aqueous populations seen in the EPR spectra. Binding a full complement of Pb2+ ions results in the stable insertion of R1 into the membrane hydrocarbon region, with average distances of 3.6 Å (173R1, C2A) and 1.7 Å (304R1, C2B) below the phosphate level.
Table 1.
Power Saturation Data for Site 173R1 and 304R1 when Syt1 Is Bound to Membranes in the Presence of Pb2+
| Label | Lipid | Metal Ion Addeda | Depth Parameter (Φ) | Position (Å)b |
|---|---|---|---|---|
| FL-SYT 173R1 | PC/PS (15%) | Pb2+ 2:1 (Pb2+:Syt1) | −1.56 ± 0.1 | −0.78 |
| Pb2+ excess | −0.72 ± 0.05 | 3.59 | ||
| FL-SYT 304R1 | PC/PS (15%) | Pb2+ 2:1 (Pb2+:Syt1) | −1.75 ± 0.1 | −3.53 |
| Pb2+ excess | −1.19 ± 0.1 | 1.73 |
Data for the 2:1 Pb2+ to FL-Syt1 ratio (1:1 lead to C2 domain) were taken to examine membrane penetration for a partially bound lead state, in which only site 1 is populated (26). In this state, there are two components in the EPR spectra, and the apparent depth has contributions from both these states. Excess lead was then added to examine differences in membrane binding when both sites are populated in the C2 domains.
The position indicates the average location of the nitroxide relative to the lipid phosphate plane. A negative value for the position indicates that the label is located on the aqueous side of the phosphate plane.
Both the EPR line shapes and the membrane depth measurements support a more peripheral and dynamic membrane-bound state of Syt1 when only site 1 is populated by the metal ion. In the full-length protein, the C2 domains are tethered to membranes via the N-terminal transmembrane region, and this facilitates the formation of such a state by creating effectively high local lipid concentrations in the vicinity of the C2 domains. Furthermore, it “primes” Syt1 to further metal ion binding events by placing its C2 domains in close proximity to the negatively charged membrane interface.
Discussion
Ca2+-dependent membrane binding of Syt1 plays a crucial role in the excitation-secretion coupling by enabling the SNAREs to initiate vesicle fusion (7,51,52). Syt1-mediated evoked release has been detected at intracellular Ca2+ concentrations as low as 25 μM (53). Surprisingly, Syt1 is an intrinsically weak Ca2+ sensor, with affinities ranging from 50 μM to >10 mM for the five Ca2+-binding sites (11, 12, 13). In the presence of anionic membranes, Ca2+ binding to Syt1 becomes highly cooperative with an apparent affinity of ∼3–4 μM, which corresponds to an ∼60–80-fold increase compared with the isolated C2AB fragment in absence of lipids (13,45). Conversely, Ca2+ binding to the Syt1 C2 domains increases their affinity to anionic membranes (45). The contribution of anionic phospholipids to this mutually cooperative response is attributed to the following: 1) masking of the positively charged surface patches of C2 (e.g., the LRC) through transient interactions, and 2) direct coordination of the C2-bound divalent metal ions by the lipid headgroups (37,38). However, the role of individual Ca2+-binding sites, specifically the high-affinity site 1 of the C2 domains, in driving the membrane interactions remained unclear. Pb2+, a high-affinity structural and functional surrogate of Ca2+, enabled us to probe the properties of the C2 domains in which site 1 is selectively populated by a divalent metal ion. We investigated the behavior of the isolated C2 domains, the C2AB fragment, and FL-Syt1.
Apart from the clear changes in electrostatics, the population of site 1 by Pb2+ alters the dynamic behavior of the membrane-binding regions. Both C2 domains in the metal-free state have a highly dynamic loop 3 that undergoes conformational exchange on a timescale <100 μs (Fig. 2). The attenuation of loop 3 dynamics caused by a metal ion at site 1 (Fig. 3) could facilitate the metal ion-dependent membrane binding for the following three reasons: 1) loop 3 provides half of oxygen ligands of the metal ion at site 1 and >60% of the coordinating ligands required for metal ion at site 2, 2) loop 3 harbors key positively charged residues (R233 in C2A, and K366 in C2B) that can potentially interact with anionic lipid headgroups upon partial or full neutralization of the intra-loop region (16,54,55), and 3) loop 3 has two hydrophobic residues (F231 and F234 for C2A, Y364 and I367 in C2B) that can potentially interact with the hydrophobic part of the bilayer (10,16,56). In addition to loop 3, the LRC region in apo C2A, but not apo C2B, shows conformational dynamics that gets attenuated by metal ion binding to site 1. The allosteric nature of this effect is surprising as the LRC is positioned >10 Å away from site 1. The functional significance of these changes in C2A dynamics remains to be investigated.
With respect to the membrane-binding properties, Pb2+ in site 1 enhances the membrane interactions of the C2 domains, individually and in the C2AB fragment (Figs. 4, 5, 6, and 7). In C2A, it serves to promote weak association with anionic membranes containing PtdSer or both PtdSer and PtdIns(4,5)P2. In C2B, Pb2+ in site 1 strengthens its metal ion-independent association with PtdIns(4,5)P2 (Fig. 6). There was no obvious manifestation of avidity with respect to membrane binding of the Pb2+-complexed C2AB fragment (Fig. 7), suggesting that either the linker region connecting the two domains is sufficiently flexible for the domains to behave independently or the C2 domains bind in “trans” mode relative to each other.
The experiments on FL-Syt1, where the protein is tethered to the membrane via the N-terminal helical segment, provided the clearest picture of the effect that sequential population of metal ion binding sites has on the membrane interactions of the C2 domains. The EPR data (Fig. 8; Table 1) show a clear correlation between the number of metal sites populated in the intra-loop region and the depth of membrane insertion. When only site 1 in both domains is occupied, Syt1 adopts a membrane-bound state that is dynamic in which the domains exchange between membrane-associated and aqueous states. The domains become completely membrane inserted in the fully metal ion-loaded state.
We speculate that the formation of this dynamic membrane-bound state of Syt1 could occur during the initial stages of membrane recruitment, when cytosolic Ca2+ predominantly populates high-affinity binding sites of the C2 domains before their binding to PtdSer. Both domains would make membrane contact, C2A through the loop regions, and C2B through its LRC region, with the latter interaction enhanced but not driven by the metal ion in site 1 (Fig. 9). The attenuated conformational dynamics would serve to preorganize the loops for subsequent metal ion- and membrane-binding events. Given high local concentration of anionic lipids (57,58) and Ca2+ (59,60) at the presynaptic membranes, the C2 domains would therefore be “primed” to bind a full complement of Ca2+ ions. This will ensure a cooperative response that is necessary for Syt1 to respond to physiological Ca2+ concentrations.
Figure 9.
Contribution of site 1 to Syt1-membrane interactions. In the metal ion-free state, only the C2B domain is interacting with the PtdIns(4,5)P2 component of the presynaptic membrane; there are no interactions with PtdSer through the loop regions of either C2 domain (SNAREs or interactions of C2B domain with SNAREs are not depicted for simplicity). Binding of metal ion to site 1 is sufficient to drive weak association of C2A with the anionic lipids of the membrane and enhance the C2B-PtdIns(4,5)P2 interactions. Both C2 domains would adopt a membrane-bound state, in which the conformational flexibility of the loop regions (and the LRC region of C2A) is attenuated. The proximity of “primed” C2 domains to anionic phospholipids ensures a mutually cooperative response, leading to the formation of fully metal ion-saturated C2 domains that undergo deep membrane insertion. To see this figure in color, go online.
Attenuation of conformational dynamics and membrane recruitment through the involvement of the highest-affinity metal ion binding site could be a feature shared by other Ca2+-dependent C2 domains. For example, stopped-flow kinetics measurements conducted by Falke’s laboratory provided evidence for the membrane binding of the C2 domain from protein kinase Cα that is complexed to a single Ca2+ ion (61). The conformational dynamics of this C2 domain upon population of site 1 is attenuated in a manner similar to that of the Syt1 C2 domains (34). Given that C2 domains are found in >100 proteins (62), the weak membrane recruitment step facilitated by a divalent metal ion at the highest-affinity site could be part of the common mechanism employed by these proteins to fulfill their membrane-associated functions.
Author Contributions
T.I.I., D.S.C., S.K., and S.B.N. designed the study. S.K., S.B.N., and B.H. conducted the experiments. S.K. and S.B.N. analyzed the data. T.I.I., S.K., D.S.C., and S.B.N. wrote the manuscript.
Acknowledgments
This work was supported by National Institutes of Health grants R01 GM108998 (to T.I.I.) and PO1 GM072694 (to D.S.C.). S.K. was supported in part by the National Science Foundation grant CHE-1905116 (to T.I.I.) and Welch Foundation grant A-1784 (to T.I.I.).
Editor: Joseph Falke.
Footnotes
Supporting Material can be found online at https://doi.org/10.1016/j.bpj.2020.01.032.
Supporting Material
References
- 1.Neher E., Sakaba T. Multiple roles of calcium ions in the regulation of neurotransmitter release. Neuron. 2008;59:861–872. doi: 10.1016/j.neuron.2008.08.019. [DOI] [PubMed] [Google Scholar]
- 2.Perin M.S., Brose N., Südhof T.C. Domain structure of synaptotagmin (p65) J. Biol. Chem. 1991;266:623–629. [PubMed] [Google Scholar]
- 3.Brose N., Petrenko A.G., Jahn R. Synaptotagmin: a calcium sensor on the synaptic vesicle surface. Science. 1992;256:1021–1025. doi: 10.1126/science.1589771. [DOI] [PubMed] [Google Scholar]
- 4.Geppert M., Goda Y., Südhof T.C. Synaptotagmin I: a major Ca2+ sensor for transmitter release at a central synapse. Cell. 1994;79:717–727. doi: 10.1016/0092-8674(94)90556-8. [DOI] [PubMed] [Google Scholar]
- 5.Zhou Q., Lai Y., Brunger A.T. Architecture of the synaptotagmin-SNARE machinery for neuronal exocytosis. Nature. 2015;525:62–67. doi: 10.1038/nature14975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brewer K.D., Bacaj T., Rizo J. Dynamic binding mode of a Synaptotagmin-1-SNARE complex in solution. Nat. Struct. Mol. Biol. 2015;22:555–564. doi: 10.1038/nsmb.3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou Q., Zhou P., Brunger A.T. The primed SNARE-complexin-synaptotagmin complex for neuronal exocytosis. Nature. 2017;548:420–425. doi: 10.1038/nature23484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Südhof T.C. A molecular machine for neurotransmitter release: synaptotagmin and beyond. Nat. Med. 2013;19:1227–1231. doi: 10.1038/nm.3338. [DOI] [PubMed] [Google Scholar]
- 9.Bai J., Wang C.T., Chapman E.R. Fusion pore dynamics are regulated by synaptotagmin∗t-SNARE interactions. Neuron. 2004;41:929–942. doi: 10.1016/s0896-6273(04)00117-5. [DOI] [PubMed] [Google Scholar]
- 10.Lynch K.L., Gerona R.R., Martin T.F. Synaptotagmin-1 utilizes membrane bending and SNARE binding to drive fusion pore expansion. Mol. Biol. Cell. 2008;19:5093–5103. doi: 10.1091/mbc.E08-03-0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ubach J., Zhang X., Rizo J. Ca2+ binding to synaptotagmin: how many Ca2+ ions bind to the tip of a C2-domain? EMBO J. 1998;17:3921–3930. doi: 10.1093/emboj/17.14.3921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fernandez I., Araç D., Rizo J. Three-dimensional structure of the synaptotagmin 1 C2B-domain: synaptotagmin 1 as a phospholipid binding machine. Neuron. 2001;32:1057–1069. doi: 10.1016/s0896-6273(01)00548-7. [DOI] [PubMed] [Google Scholar]
- 13.Fernández-Chacón R., Königstorfer A., Südhof T.C. Synaptotagmin I functions as a calcium regulator of release probability. Nature. 2001;410:41–49. doi: 10.1038/35065004. [DOI] [PubMed] [Google Scholar]
- 14.Fernández-Chacón R., Shin O.H., Rosenmund C. Structure/function analysis of Ca2+ binding to the C2A domain of synaptotagmin 1. J. Neurosci. 2002;22:8438–8446. doi: 10.1523/JNEUROSCI.22-19-08438.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Radhakrishnan A., Stein A., Fasshauer D. The Ca2+ affinity of synaptotagmin 1 is markedly increased by a specific interaction of its C2B domain with phosphatidylinositol 4,5-bisphosphate. J. Biol. Chem. 2009;284:25749–25760. doi: 10.1074/jbc.M109.042499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang X., Rizo J., Südhof T.C. Mechanism of phospholipid binding by the C2A-domain of synaptotagmin I. Biochemistry. 1998;37:12395–12403. doi: 10.1021/bi9807512. [DOI] [PubMed] [Google Scholar]
- 17.Araç D., Chen X., Rizo J. Close membrane-membrane proximity induced by Ca(2+)-dependent multivalent binding of synaptotagmin-1 to phospholipids. Nat. Struct. Mol. Biol. 2006;13:209–217. doi: 10.1038/nsmb1056. [DOI] [PubMed] [Google Scholar]
- 18.Kuo W., Herrick D.Z., Cafiso D.S. Phosphatidylinositol 4,5-bisphosphate alters synaptotagmin 1 membrane docking and drives opposing bilayers closer together. Biochemistry. 2011;50:2633–2641. doi: 10.1021/bi200049c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang S., Li Y., Ma C. Synaptotagmin-1 C2B domain interacts simultaneously with SNAREs and membranes to promote membrane fusion. eLife. 2016;5:e14211. doi: 10.7554/eLife.14211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gorkhali R., Huang K., Yang J.J. Defining potential roles of Pb2+ in neurotoxicity from a calciomics approach. Metallomics. 2016;8:563–578. doi: 10.1039/c6mt00038j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Katti S., Nyenhuis S.B., Igumenova T.I. Non-native metal ion reveals the role of electrostatics in Synaptotagmin 1-membrane interactions. Biochemistry. 2017;56:3283–3295. doi: 10.1021/acs.biochem.7b00188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rajalingam D., Kumar T.K., Yu C. The C2A domain of synaptotagmin exhibits a high binding affinity for copper: implications in the formation of the multiprotein FGF release complex. Biochemistry. 2005;44:14431–14442. doi: 10.1021/bi051387r. [DOI] [PubMed] [Google Scholar]
- 23.Morales K.A., Lasagna M., Igumenova T.I. Pb2+ as modulator of protein-membrane interactions. J. Am. Chem. Soc. 2011;133:10599–10611. doi: 10.1021/ja2032772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morales K.A., Yang Y., Igumenova T.I. Cd2+ as a Ca2+ surrogate in protein-membrane interactions: isostructural but not isofunctional. J. Am. Chem. Soc. 2013;135:12980–12983. doi: 10.1021/ja406958k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morales K.A., Igumenova T.I. Synergistic effect of Pb(2+) and phosphatidylinositol 4,5-bisphosphate on C2 domain-membrane interactions. Biochemistry. 2012;51:3349–3360. doi: 10.1021/bi201850h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Katti S., Her B., Igumenova T.I. High affinity interactions of Pb2+ with synaptotagmin I. Metallomics. 2018;10:1211–1222. doi: 10.1039/c8mt00135a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nyenhuis S.B., Thapa A., Cafiso D.S. Phosphatidylinositol 4,5 bisphosphate controls the cis and trans interactions of Synaptotagmin 1. Biophys. J. 2019;117:247–257. doi: 10.1016/j.bpj.2019.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.King E.J. The colorimetric determination of phosphorus. Biochem. J. 1932;26:292–297. doi: 10.1042/bj0260292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Delaglio F., Grzesiek S., Bax A. NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J. Biomol. NMR. 1995;6:277–293. doi: 10.1007/BF00197809. [DOI] [PubMed] [Google Scholar]
- 30.Lee W., Tonelli M., Markley J.L. NMRFAM-SPARKY: enhanced software for biomolecular NMR spectroscopy. Bioinformatics. 2015;31:1325–1327. doi: 10.1093/bioinformatics/btu830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Farrow N.A., Muhandiram R., Kay L.E. Backbone dynamics of a free and phosphopeptide-complexed Src homology 2 domain studied by 15N NMR relaxation. Biochemistry. 1994;33:5984–6003. doi: 10.1021/bi00185a040. [DOI] [PubMed] [Google Scholar]
- 32.Wang L., Pang Y., Zuiderweg E.R. Functional dynamics in the active site of the ribonuclease binase. Proc. Natl. Acad. Sci. USA. 2001;98:7684–7689. doi: 10.1073/pnas.121069998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Frazier A.A., Roller C.R., Cafiso D.S. Membrane-bound orientation and position of the synaptotagmin I C2A domain by site-directed spin labeling. Biochemistry. 2003;42:96–105. doi: 10.1021/bi0268145. [DOI] [PubMed] [Google Scholar]
- 34.Morales K.A., Yang Y., Igumenova T.I. Dynamic response of the C2 domain of protein kinase Cα to Ca2+ binding. Biophys. J. 2016;111:1655–1667. doi: 10.1016/j.bpj.2016.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pérez-Lara Á., Thapa A., Jahn R. PtdInsP2 and PtdSer cooperate to trap synaptotagmin-1 to the plasma membrane in the presence of calcium. eLife. 2016;5:e15886. doi: 10.7554/eLife.15886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Millet O., Bernadó P., Pons M. NMR measurement of the off rate from the first calcium-binding site of the synaptotagmin I C2A domain. FEBS Lett. 2002;516:93–96. doi: 10.1016/s0014-5793(02)02508-5. [DOI] [PubMed] [Google Scholar]
- 37.Honigmann A., van den Bogaart G., Jahn R. Phosphatidylinositol 4,5-bisphosphate clusters act as molecular beacons for vesicle recruitment. Nat. Struct. Mol. Biol. 2013;20:679–686. doi: 10.1038/nsmb.2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Verdaguer N., Corbalan-Garcia S., Gómez-Fernández J.C. Ca(2+) bridges the C2 membrane-binding domain of protein kinase Calpha directly to phosphatidylserine. EMBO J. 1999;18:6329–6338. doi: 10.1093/emboj/18.22.6329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kuo W., Herrick D.Z., Cafiso D.S. The calcium-dependent and calcium-independent membrane binding of synaptotagmin 1: two modes of C2B binding. J. Mol. Biol. 2009;387:284–294. doi: 10.1016/j.jmb.2009.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xue M., Ma C., Rizo J. The Janus-faced nature of the C(2)B domain is fundamental for synaptotagmin-1 function. Nat. Struct. Mol. Biol. 2008;15:1160–1168. doi: 10.1038/nsmb.1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guillén J., Ferrer-Orta C., Corbalán-García S. Structural insights into the Ca2+ and PI(4,5)P2 binding modes of the C2 domains of rabphilin 3A and synaptotagmin 1. Proc. Natl. Acad. Sci. USA. 2013;110:20503–20508. doi: 10.1073/pnas.1316179110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fukuda M., Moreira J.E., Llinás R. Role of the C2B domain of synaptotagmin in vesicular release and recycling as determined by specific antibody injection into the squid giant synapse preterminal. Proc. Natl. Acad. Sci. USA. 1995;92:10708–10712. doi: 10.1073/pnas.92.23.10708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chapman E.R., Desai R.C., Tornehl C.K. Delineation of the oligomerization, AP-2 binding, and synprint binding region of the C2B domain of synaptotagmin. J. Biol. Chem. 1998;273:32966–32972. doi: 10.1074/jbc.273.49.32966. [DOI] [PubMed] [Google Scholar]
- 44.Bai J., Tucker W.C., Chapman E.R. PIP2 increases the speed of response of synaptotagmin and steers its membrane-penetration activity toward the plasma membrane. Nat. Struct. Mol. Biol. 2004;11:36–44. doi: 10.1038/nsmb709. [DOI] [PubMed] [Google Scholar]
- 45.van den Bogaart G., Meyenberg K., Jahn R. Phosphatidylinositol 4,5-bisphosphate increases Ca2+ affinity of synaptotagmin-1 by 40-fold. J. Biol. Chem. 2012;287:16447–16453. doi: 10.1074/jbc.M112.343418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guerrero-Valero M., Ferrer-Orta C., Corbalán-García S. Structural and mechanistic insights into the association of PKCalpha-C2 domain to PtdIns(4,5)P2. Proc. Natl. Acad. Sci. USA. 2009;106:6603–6607. doi: 10.1073/pnas.0813099106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chapman E.R., Jahn R. Calcium-dependent interaction of the cytoplasmic region of synaptotagmin with membranes. Autonomous function of a single C2-homologous domain. J. Biol. Chem. 1994;269:5735–5741. [PubMed] [Google Scholar]
- 48.Fuson K.L., Montes M., Sutton R.B. Structure of human synaptotagmin 1 C2AB in the absence of Ca2+ reveals a novel domain association. Biochemistry. 2007;46:13041–13048. doi: 10.1021/bi701651k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu H., Bai H., Chapman E.R. Linker mutations reveal the complexity of synaptotagmin 1 action during synaptic transmission. Nat. Neurosci. 2014;17:670–677. doi: 10.1038/nn.3681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Evans C.S., He Z., Chapman E.R. Functional analysis of the interface between the tandem C2 domains of synaptotagmin-1. Mol. Biol. Cell. 2016;27:979–989. doi: 10.1091/mbc.E15-07-0503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dai H., Shen N., Rizo J. A quaternary SNARE-synaptotagmin-Ca2+-phospholipid complex in neurotransmitter release. J. Mol. Biol. 2007;367:848–863. doi: 10.1016/j.jmb.2007.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chapman E.R. How does synaptotagmin trigger neurotransmitter release? Annu. Rev. Biochem. 2008;77:615–641. doi: 10.1146/annurev.biochem.77.062005.101135. [DOI] [PubMed] [Google Scholar]
- 53.Schneggenburger R., Neher E. Intracellular calcium dependence of transmitter release rates at a fast central synapse. Nature. 2000;406:889–893. doi: 10.1038/35022702. [DOI] [PubMed] [Google Scholar]
- 54.Wang P., Wang C.T., Chapman E.R. Mutations in the effector binding loops in the C2A and C2B domains of synaptotagmin I disrupt exocytosis in a nonadditive manner. J. Biol. Chem. 2003;278:47030–47037. doi: 10.1074/jbc.M306728200. [DOI] [PubMed] [Google Scholar]
- 55.Shao X., Li C., Rizo J. Synaptotagmin-syntaxin interaction: the C2 domain as a Ca2+-dependent electrostatic switch. Neuron. 1997;18:133–142. doi: 10.1016/s0896-6273(01)80052-0. [DOI] [PubMed] [Google Scholar]
- 56.Chapman E.R., Davis A.F. Direct interaction of a Ca2+-binding loop of synaptotagmin with lipid bilayers. J. Biol. Chem. 1998;273:13995–14001. doi: 10.1074/jbc.273.22.13995. [DOI] [PubMed] [Google Scholar]
- 57.James D.J., Khodthong C., Martin T.F. Phosphatidylinositol 4,5-bisphosphate regulates SNARE-dependent membrane fusion. J. Cell Biol. 2008;182:355–366. doi: 10.1083/jcb.200801056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lauwers E., Goodchild R., Verstreken P. Membrane lipids in presynaptic function and disease. Neuron. 2016;90:11–25. doi: 10.1016/j.neuron.2016.02.033. [DOI] [PubMed] [Google Scholar]
- 59.Bornschein G., Schmidt H. Synaptotagmin Ca2+ sensors and their spatial coupling to presynaptic Cav channels in central cortical synapses. Front. Mol. Neurosci. 2019;11:494. doi: 10.3389/fnmol.2018.00494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bilkova E., Pleskot R., Coskun Ü. Calcium directly regulates phosphatidylinositol 4,5-bisphosphate headgroup conformation and recognition. J. Am. Chem. Soc. 2017;139:4019–4024. doi: 10.1021/jacs.6b11760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Corbin J.A., Evans J.H., Falke J.J. Mechanism of specific membrane targeting by C2 domains: localized pools of target lipids enhance Ca2+ affinity. Biochemistry. 2007;46:4322–4336. doi: 10.1021/bi062140c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lemmon M.A. Membrane recognition by phospholipid-binding domains. Nat. Rev. Mol. Cell Biol. 2008;9:99–111. doi: 10.1038/nrm2328. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.