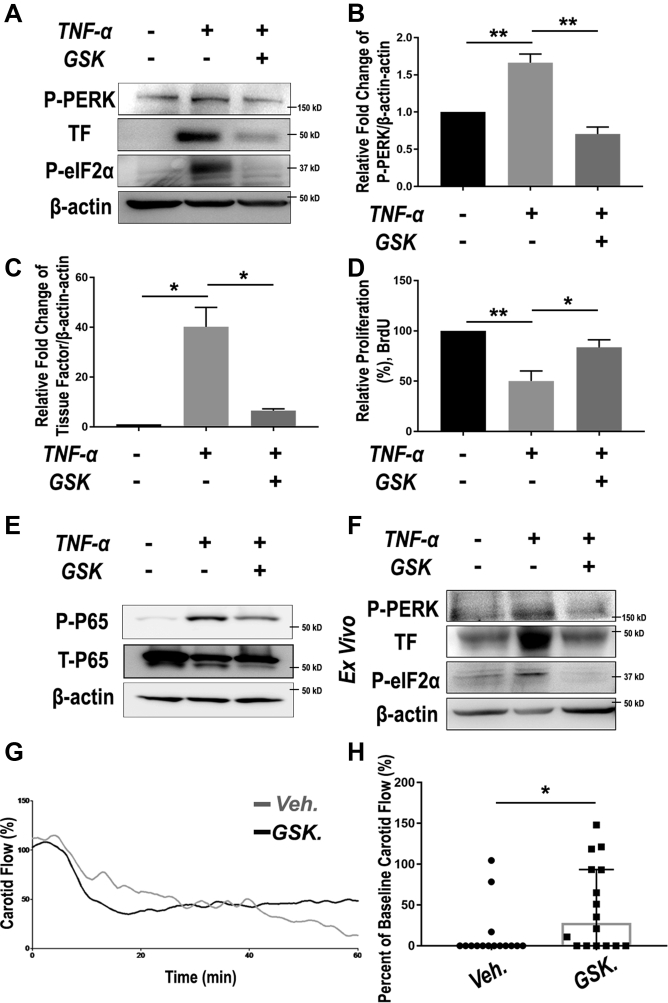
Figure 6.
PERK Inhibition Rescues EC Dysfunction and Mitigates Thrombosis
(A to E) Human primary aortic endothelial cells (ECs) were pre-treated with vehicle or 1 μmol/l GSK2606414 for 2 h before TNF-α stimulation (20 ng/ml). Cells were harvested at 6 h and 24 h after TNF-α treatment for immunoblotting and BrdU assays, respectively. p65 phosphorylation was determined 15 min after TNF-α stimulation. (A) Representative immunoblots of PERK pathway (P-PERK and P-eIF2α) and EC dysfunction marker (thrombogenic marker tissue factor [TF]). (B and C) Quantitation of PERK phosphorylation level and TF expression in ECs stimulated with PDGF-BB and PERK inhibitor pre-treatment. Protein band densitometry was normalized to β-actin; n = 3. (D) Proliferation of ECs was determined using BrdU incorporation enzyme-linked immunosorbent assay; n = 3. Data in B to D are presented as mean ± SEM, *p < 0.05, **p < 0.01, 1-way analysis of variance with Bonferroni post hoc test. (E) Representative immunoblots of NFκB pathway (p65 phosphorylation). Shown are representative blots from 2 similar experiments. (F) Ex vivo PERK pathway activation by TNF-α and its blockade by pretreatment with GSK2606414. Cultured rat aortic rings were pre-treated with vehicle or 1 μmol/l GSK2606414 for 2 h before a 6-h stimulation with TNF-α (20 ng/ml). Shown are representative blots from 2 similar experiments. (G and H) Mice were pre-treated with either vehicle or GSK2606414 (150 mg/kg) via oral gavage for 4 h, and then subjected to FeCl3 topical application to induce carotid artery thrombosis. (G) Real-time recording of blood velocity in FeCl3-injured carotid arteries. (H) Scatter plot of arterial flow at the end of the 60-min recording. A 0% flow indicates complete occlusion due to thrombosis. Median with interquartile range, n = 15 to 16 mice. *p < 0.05, Mann-Whitney nonparametric test. Abbreviations as in Figure 1.