Abstract
Objective: This pilot randomized controlled trial (RCT) aimed at evaluating the feasibility and potential efficacy of a motivational interviewing (MI) intervention to increase physical activity (PA) behavior in cancer patients. Methods: Participants were randomly assigned to an experimental group with standard care plus 12 MI sessions within 12 weeks or a control group with standard care only. The number of recruited participants and the modality of recruitment were recorded to describe the reach of the study. The acceptability of the study was estimated using the attrition rate during the intervention phase. The potential efficacy of the intervention was evaluated by analyzing the PA behavior. Results: Twenty-five participants were recruited within the 16-month recruitment period (1.6 participants per month). Five participants (38.5%) from the experimental group (n = 13) and one participant (8.3%) from the control group (n = 12) dropped out of the study before the end of the intervention phase. No group by time interaction effect for PA behavior was observed at the end of the intervention. Conclusion: Due to the low recruitment rate and compliance, no conclusion can be drawn regarding the efficacy of MI to increase PA behavior in cancer patients. Moreover, the current literature cannot provide any evidence on the effectiveness of MI to increase PA in cancer survivors. Future RCTs should consider that the percentage of uninterested patients to join the study may be as high as 60%. Overrecruitment (30% to 40%) is also recommended to accommodate the elevated attrition rate.
Keywords: cancer, physical activity, motivation, accelerometry, fitness
Introduction
Postdiagnosis physical activity (PA) reduces all-cause and cancer-specific mortality especially in breast cancer, colorectal cancer, and endometrium cancer survivors.1-5 PA is not only efficient in reducing the risk of cancer events, such as cancer progression, new primaries, and cancer recurrence, but it also increases/maintains physical fitness, including cardiorespiratory fitness and muscle strength.6-9 It has significant positive effects on several cancer-related symptoms including fatigue, sleep disturbance, depression, and anxiety.10-12 PA, therefore, contributes significantly to improve the quality of life in cancer patients.11,13 Notwithstanding the existing evidence on the health benefits of PA in cancer patients and PA recommendations,14 PA engagement and levels are generally low. Population-based studies have found that between 22% and 78% of cancer patients meet the minimal World Health Organization’s guidelines.15-19 More precisely, Barker et al showed that cancer survivors had a 1.14 hours lower weekly moderate-to-vigorous PA (MVPA) engagement than individuals without noncommunicable diseases.20 Typically, cancer patients decrease their PA commitment as their treatment progresses21,22 as well as within the first years after treatment.16
Cancer patients require significant motivation and encouragement to engage in regular PA or maintain their PA levels during and after treatment.23 PA promotion in health care, and especially PA prescription by medical doctors, is a first step to engage patients into a more active lifestyle.24 Clinicians are encouraged to assess PA of their patients with cancer, to provide information and advice on the subject, and refer them to the appropriate professionals to engage more in an active lifestyle.25 Nevertheless, even if oncologists are able to provide relevant PA recommendations in clinical routine,26 this may still not be sufficient for patients to increase their PA levels.27 However, if the oncologists’ recommendations are supported by motivational arguments and specific information regarding PA opportunities, the odds for greater PA engagement are increased.13 Complementary behavior change strategies have been shown to increase PA engagement by more than 1 hour per week in cancer patients.28,29 One promising strategy is motivational interviewing (MI), a patient-centered approach to develop the motivation of patients for behavioral change through open-ended discussions.30 MI aims to create behavior change by allowing patients to identify their own reasons for wanting to change a behavior. MI is widely promoted across the medical field to address a variety of behavioral targets, including change in PA behavior. MI may have several advantages for its rapid implementation in the health care system. First, it may be effectively delivered by most health and/or exercise professionals following a minimum of training.31 This technique appears to require less contact hours of treatment compared with other behavior change strategies.31,32 Finally, MI is well accepted by patients due to its person-centered nature.33 Systematic reviews and meta-analyses showed that MI moderately increased PA behaviors in individuals with noncommunicable diseases (ie, obesity, diabetes, cardiovascular diseases, multiple sclerosis, fibromyalgia).34-37 MI seems so promising in changing PA behavior that its use is even recommended following the PA prescription/referral for insufficiently active people with noncommunicable diseases (including cancer) in the United Kingdom, France, and Sweden.38-40 To our knowledge, 2 literature reviews concluded that MI counseling was appropriate to change PA behaviors in cancer patients.41,42 Based on these literature reviews and our own literature search, we identified a total of 9 studies that evaluated MI interventions to increase PA in cancer patients.43-51 Three of them concluded that MI intervention aiming to improve PA was well accepted by cancer patients.43,46,48 However, among these 9 studies, 5 were randomized controlled trials (RCTs) evaluating PA behavior directly (ie, not via a proxy of PA) and showed inconsistent results.44-48 Furthermore, only 2 studies measured objective PA behavior by means of an accelerometer. Therefore, we aimed at running a pilot study to assess the feasibility of an MI intervention to increase objectively evaluated PA behavior (using hip-worn 3-dimensional [3D] accelerometers), physical fitness, and quality of life in cancer patients.
Materials and Methods
Study Design
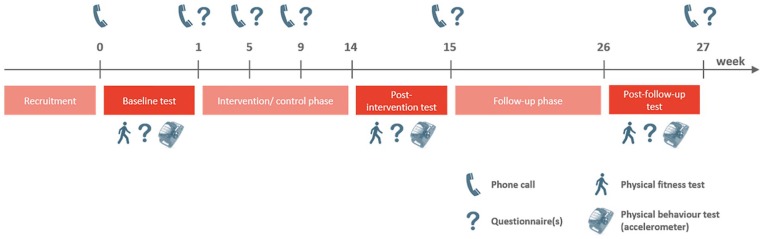
This pilot study was designed as an RCT. Procedures were approved by the National Ethics Committee (No. 201704/02 version 1.3) and declared on www.clinicaltrials.gov (No. NCT03210129). The study phase lasted 6 months, including an intervention/control period and a follow-up period of 3 months each (Figure 1). Each participant read and signed an informed consent form during the first visit at baseline. All participants received information regarding the PA recommendations and the national PA program for cancer patients via a dedicated website (www.sport-sante.lu). Participants completed a demographic questionnaire and performed anthropometric and physical fitness measurements. For the assessment of PA behavior, participants received a 3D accelerometer, which they were asked to wear for the upcoming week. One week after the baseline phase, participants were randomly assigned to the control or experimental group. During the first 3 months, participants assigned to the experimental group attended 1 MI session per week. The control group did not receive any additional intervention besides their standard care. After the intervention/control phase (at 3 months) and the follow-up phase (at 6 months after entering the trial), the same measurements were performed except for the background questionnaire. Quality of life was investigated at baseline and after 1, 2, 3, and 6 months.
Figure 1.
Timeline of the study procedure for all participants.
Participants
Eligible participants were nonmetastatic cancer (breast, endometrial, or colorectal) patients, aged over 18 years, who had completed the treatment (immediate study inclusion after chemotherapy and/or radiotherapy or, if applicable, inclusion 3 months after surgery) up to 2 years before inclusion. Additionally, participants had to be cleared to engage in regular PA by their oncologist. Exclusion criteria were a second primary tumor, recurrent cancer, a history of other cancer types, a known or obvious cognitive impairment or mental disorder, pregnancy, or metastatic cancer.
Recruitment
Study recruitment lasted 16 months (from September 2017 to December 2018). The study was presented in 3 different Luxembourgish hospitals where at least 21 oncologists worked. The clinicians were kindly invited to inform eligible patients of the study. Flyers of the study were provided to the oncology department of these hospitals as well as 2 obstetrics and gynecology clinics who were kindly asked to hand them out to eligible patients. Furthermore, the study was promoted at 3 community events as well as by 20 publications in the local general and specialized press and on their social network platforms or websites.
Motivational Interviewing Intervention
Participants who were assigned to the experimental group took part in 12 MI sessions over the 12-week intervention phase. The first and seventh sessions were individual face-to-face interviews, while the remaining 10 meetings took place over the phone. Participants were required to take part in a minimum of 10 sessions to validate the intervention. Two research assistants, who were not initially trained in any behavior change technique, followed a training in MI techniques (21 hours) and carried out these interviews. The MI training consisted of a 3-day workshop on MI delivered by a certified MI trainer from the “Association francophone de diffusion de l’entretien motivationnel.” The first MI session involved an encouraging discussion on the health benefits associated with PA, which was intended to identify the participants’ current PA behaviors and beliefs. Based on this, strategies to overcome ambivalence about PA were developed and included goal setting, problem solving, addressing confidence, addressing beliefs about PA, and encouragements. When appropriate, the counselor offered advice and support for participants who were not meeting their goals and suggested areas for further behavior changes that could be more effective.
Outcome Variables
The aim of the study was to evaluate the feasibility of the MI intervention to increase PA behavior measured with accelerometers, physical fitness, and quality of life in cancer patients. The evaluation of the potential efficacy of the MI intervention was a secondary objective.
Feasibility
The number of recruited patients and the modality of recruitment (ie, oncologist, flyers, etc) were recorded to describe the reach of the study. The acceptability of the study was estimated based on the attrition rate through the intervention and the follow-up phase. The number of completed MI sessions was also recorded.
Potential Efficacy
The potential efficacy (ie, limited-efficacy testing52) of the MI intervention was evaluated by analyzing the following outcomes: PA behavior, body mass index (BMI), physical fitness, and quality of life.
PA behavior
MVPA was assessed using accelerometry and used as primary outcome variable to evaluate the potential efficacy of the present pilot study. The accelerometer (ActiGraphTM GT3X+, Pensacola, FL) was worn on the hip on the side of the nondominant hand, collecting triaxial acceleration data with a sampling frequency of 100 Hz and a dynamic range of ±8 g. Participants were asked to wear the device continuously for 1 week, except when showering and during water activities (eg, swimming). Nonwear periods were requested to be marked in a diary log, indicating time and reason for nonwear. The raw data collected by the accelerometer were extracted using the ActiLife software (version 6.13.3) and subsequently imported into STATA (version SE 15.1) for processing using the mean amplitude deviation (MAD) approach.53 This approach consists in the computation of the resultant acceleration (r) for each time point using the following equation: where AccX, AccY, and AccZ are the raw acceleration signals for the 3 different axes. The mean resultant acceleration (R) is calculated over epoch lengths of 5 seconds, and the absolute deviation (abs_dev) for each time point is calculated using the equation: Last, the MAD is generated by calculating the average of the absolute deviation over a time period of 5 seconds.
Compliance was operationalized as the total amount of wear time. A period was defined as nonwear as soon as the MAD was equal to 0 for 60 consecutive minutes.54 In the present study, the nonwear periods were not imputed with calculated data, to ensure fully objective data. Based on the MAD values, the total time spent at different PA intensities was defined using the following thresholds: no movement: MAD = 0 mg, sedentary behavior: 0 mg < MAD ≤ 22.5 mg, light PA: 22.5 mg < MAD ≤ 91 mg, moderate PA (MPA): 91 mg < MAD ≤ 414 mg, vigorous PA (VPA): MAD > 414 mg.55,56 The MVPA category represents the sum of the time spent in MPA and VPA. The total time spent in each category was calculated for each day and for the whole week (7 days). Data were analyzed only if there were valid recordings over at least 4 days, including 1 weekend day. A day was rated as valid if the wear time was longer than 10 hours.57
Body mass index
Height and weight were measured during each visit, using a height gauge and a body weighing scale (SECA, Model 763) to calculate BMI.
Physical fitness
Physical fitness was evaluated using 3 different tests performed in a specific order. (1) The 30-second chair-rise test was used to evaluate leg strength and involved the participants rising to a full standing position and sitting back down again as often as possible within 30 seconds.58 The total number of repetitions was retained. (2) The Southampton protocol was used to evaluate handgrip strength.59 A Jamar handgrip dynamometer (Model 5030J1) was used to measure the maximal isometric handgrip strength of both hands. Each hand was measured 3 times, with the best of the 6 results being retained for statistical analysis. (3) For the assessment of cardiovascular fitness, participants were asked to perform an incremental walking test on a treadmill (Woodway, PPS70 Plus, Germany), using the Ramped Bruce Protocol.60 The test started with an initial speed of 1.6 km/h and no slope. The speed and slope were progressively increased according to the stages of the predefined protocol. The test ended as soon as a heart rate of 85% of the age-adjusted estimate of the maximal heart rate () was reached.61 Heart rate was continuously measured using a chest strap heart rate sensor from Polar (Model T34). The last completed stage (ranging from 1 to 60) of the Ramped Bruce Protocol at the targeted heart rate was retained for further analysis.
Quality of life
Quality of life was evaluated using the EQ-5D-5L questionnaire, which is a generic questionnaire consisting of 6 items.62 The first 5 items evaluate the individual’s health in 5 dimensions (mobility, self-care, usual activities, pain/discomfort, and anxiety/depression) using 5 levels of answer for each dimension. These 5D health profiles were transformed into single index values, ranging from 0 (poor health) to 1 (good health). The sixth item of the instrument evaluated perceived health using the Visual Analogue Scale (VAS) ranging from 0 (poor health) to 100 (good health).
Sample Size
The aim of this pilot study was to examine the feasibility and acceptability of the protocol, as well as the identification of unforeseen problems, which could compromise a subsequent larger investigation. To investigate these unforeseen problems, which may manifest in at least 1 of 20 patients (5 %, π = 0.05) at a confidence level of 95% (γ = 0.95), a total of 59 participants was required based on the following equation63:
To further account for a 20% dropout, a total of 70 patients should have been enrolled in the study (ie, 35 patients in each group).
Randomization
Every participant was given a person-specific code, and all information collected was pseudonymized. Participants were randomly assigned to the experimental group with MI or the control group. Group assignment was carried out by a MI counselor using randomization lists generated by the Competence Center in Methodology and Statistics (Luxembourg Institute of Health). For each type of cancer, a separate randomization list was used. The group assignment was hidden to the investigators and to the scientific collaborators who collected, recorded, and analyzed the data. However, both participants and the 2 MI counselors were aware of the group assignment.
Statistical Analysis
Categorical data were expressed as number and percentage. Group comparisons of participants’ personal characteristics were performed using χ2 test or Fisher’s exact test for categorical data, depending on test assumptions. Continuous variables were expressed as median with the first and third quartiles. The assumption of normality of distribution was tested using Shapiro-Wilk test. Unpaired t tests or Mann-Whitney U tests were used for continuous variables, depending on normality of distribution, respectively. Effects of the intervention on PA behavior, BMI, physical fitness, and quality of life were investigated using a linear mixed-effect model with restricted maximum likelihood estimation. Separate mixed-effect models with subject-specific random intercept were defined to analyze each variable of interest. Accordingly, the participant was included as random effect to adjust for within-subject correlation over time (different intercepts for each subject). The group allocation, the measurement time point, and the group by time interaction were defined as fixed effects. These 3 predictors were sufficient considering the low sample size and the successful and equal distribution of participants between the 2 groups by the randomization procedure. Considering the decline in overall wear time from the baseline test to the last test after 6 months, wear time was defined as covariate in the linear mixed-effect models for the variables evaluating PA behavior. Additionally, a log transformation of the cardiovascular fitness data and an ordered quantile normalizing transformation of the quality of life data were applied to improve the relative normality of the residuals. Cohen’s d effect sizes were provided for group by time interaction effects. A .05 P level of significance was set for all analyses.
Results
Feasibility
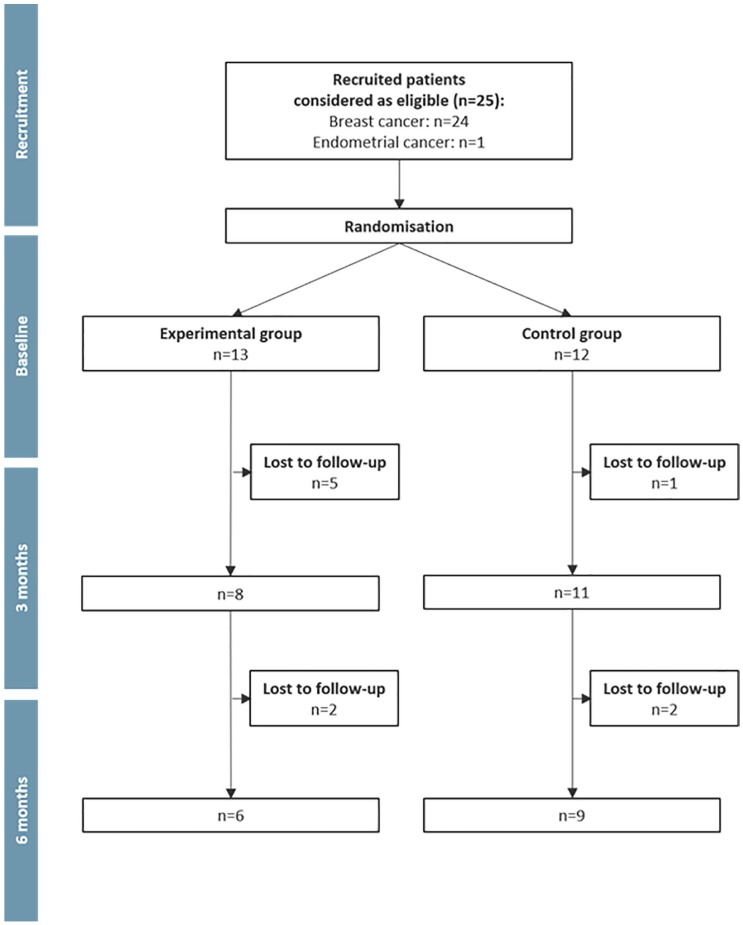
Over the planned recruitment period (16 months), 25 female cancer patients (24 with breast cancer and 1 with endometrial cancer) consented to participate in the study, performed the baseline tests, and were randomly allocated to 1 of the 2 groups (Figure 2). The recruitment rate was, therefore, 1.6 patients per month. Nineteen participants (76%) were informed of the study by 1 single oncologist, 4 participants (16%) were informed by 3 other oncologists, and 2 participants (8%) were recruited through the promotion on social network platforms. The total number of patients who were informed of the study was not recorded due to the multiple nature of the study promotion. No difference was observed between the experimental and control groups for the participants’ personal characteristics, assessed at baseline (Table 1).
Figure 2.
Flow chart of the study.
Table 1.
Characteristics of the 25 Recruited Participants (Including Those Who Dropped Out Before the End of the Intervention) at Baseline.
| Experimental Group, N = 8 |
Control Group, N = 11 |
Dropped Out Participants, N = 6 |
|
|---|---|---|---|
| Median (Q1, Q3) or n (%) | Median (Q1, Q3) or n (%) | Median (Q1, Q3) or n (%) | |
| Age (years) | 45.9 (44.6, 47.3) | 45.5 (41.7, 47.0) | 51.0 (45.4, 57.7) |
| Time since treatment (years) | 1.0 (0.7, 1.8) | 1.2 (0.6, 2.0) | 1.4 (0.6, 2.2) |
| Cancer type | |||
| Breast cancer | 7 (87%) | 11 (100%) | 6 (100%) |
| Endometrial cancer | 1 (13%) | 0 (0%) | 0 (0%) |
| Cancer stage | |||
| Stage 1 | 2 (25%) | 3 (27%) | 3 (50%) |
| Stage 2 | 0 (0%) | 3 (27%) | 1 (17%) |
| Stage 3 | 6 (75%) | 5 (46%) | 2 (33%) |
| Treatment | |||
| Surgery (yes) | 6 (75%) | 10 (91%) | 6 (100%) |
| Radiotherapy (yes) | 7 (87%) | 10 (91%) | 2 (33%) |
| Chemotherapy (yes) | 6 (75%) | 9 (82%) | 3 (50%) |
| Additional treatment | |||
| Hormone therapy | 2 (25%) | 5 (46%) | 3 (50%) |
| Immunotherapy | 0 (0%) | 1 (9%) | 1 (17%) |
| No additional treatment | 6 (75%) | 5 (46%) | 1 (17%) |
During the intervention phase, 5 participants (38.5%) from the experimental group (n = 13) and 1 participant (8.3%) from the control group (n = 12) withdrew from the study (P = .16). These dropout participants (n = 6) were older (P = .044), and fewer had radiotherapy treatment (P = .024; Table 1). The participants who withdrew from the experimental group completed 28.3% (±15.1%) of the 12 MI sessions, whereas the 8 individuals who continued participation in this group completed 86.5% (±18.1%) of the 12 MI sessions. Several participants from the experimental group thought that the MI intervention was a sort of coaching with a defined and precise PA program. For 3 participants, the MI sessions had to be postponed several times. The duration of the face-to-face MI sessions was on average 47 ± 20 minutes (48 ± 18 minutes for the dropouts), whereas the duration of the phone MI sessions was on average 24 ± 10 minutes (24 ± 11 minutes for the dropouts).
During the follow-up phase, 4 participants (2 in each group) withdrew from the study. Participants who only completed the first test were excluded from the analysis for limited-efficacy testing, as no comparison between different measurement time points was possible.
For the analysis of the PA behavior, 3 files (6% of the 53 accelerometer datasets) had to be discarded due to technical issues and 2 files (4%) did not fulfill the requirements of a valid week with regard to wear time.
Limited-Efficacy Testing
A descriptive analysis of PA behavior, anthropometrics, and physical fitness is presented as Supplementary Material 1 (available online). At baseline, participants engaged on average in 9.16 (±3.51) hours of MVPA per week (8.86 ± 3.05 hours of MPA, 0.30 ± 0.46 hours of VPA per week). When controlling for wear time, no significant group by time interaction effect was observed for MVPA (d = 0.46, P = .27) and for sedentary behavior (d = 0.12, P = .77) at 3 months (postintervention). Concerning the physical fitness, no group by time interaction effect was observed for BMI (d = 0.23, P = .54), the 30-second chair test (d = 0.21, P = .57), cardiovascular fitness (d = 0.01, P = .99), and handgrip strength (d = 0.42, P = .25) at 3 months. Supplementary Material 2 (available online) shows the results of the index and the VAS scores reflecting quality of life. No group by time interaction effect was observed for the index (d = 0.28, P = .27) and the VAS (d = 0.33, P = .19) at 3 months.
Discussion
The main objective of this pilot study was to evaluate the feasibility of a MI intervention on PA behavior engagement measured with accelerometers, physical fitness, and quality of life in cancer patients. The low recruitment and the high attrition rate in the experimental group during the intervention showed a limited feasibility and patients’ acceptability of the study protocol.
Feasibility
Yearly, more than 600 women are diagnosed with breast cancer, colorectal cancer, or endometrial cancer in Luxembourg (eg, 450 breast, 144 colorectal, and 45 endometrial cancers in 201764). It is unlikely that all patients would have been eligible to participate in the present study (eg, metastatic cancer). However, we would have expected to recruit a sufficient number of patients to meet the computed sample size. Unfortunately, only 25 patients were involved in the present study, despite increased efforts to promote it. We identified 9 studies that evaluated the MI on PA behavior in cancer patients. These studies screened between 77 and 513 patients (Table 2).43-51 The percentage of eligible patients ranged from 47% to 93% according to their inclusion and exclusion criteria. In addition, the percentage of eligible patients who were not interested in participating in such MI interventions was on average 57%, ranging from 19% to 76%. Therefore, these 9 studies had also small sample sizes, ranging from 17 to 66 participants (from 31 to 66 for the RCTs). The average recruitment period of these RCTs was 11 months (ranging from 6 and 14 months), with an average recruitment rate of 4 patients per month (ranging from 2 to 9 patients per months). In the present study, the recruitment rate was lower with 1.6 patients per month. Systematic reviews of studies evaluating MI to improve PA behavior in patients with other noncommunicable diseases (obesity, diabetes cardiovascular diseases, multiple sclerosis, fibromyalgia) reported sample sizes ranging from 19 to 1570 patients, with 19 (42%) out of 45 studies including less than 100 patients.34-37 In the studies from these systematic reviews, the percentage of screened eligible patients who were not interested in participating in such MI interventions was 60%, ranging from 6% to 77% (n = 19 studies). The average recruitment period of these RCTs was 15 months (ranging from 3 to 38 months), with an average recruitment rate of 9 patients per month (ranging from 4 to 52 patients per months; n = 18 studies; a multicentric study that included 392 patients per month was not included). Even if the percentage of the patients who are not interested in participating in an MI intervention is roughly the same across the noncommunicable diseases, the recruitment rate is more than twice lower in cancer settings. Low recruitment rates were also observed in studies evaluating the effect of other behavioral change technique interventions to increase PA levels in cancer patients, suggesting a low motivation in that population for participating in such research programs and/or more constraining inclusion/exclusion criteria.29 Four barriers were identified in the low participation rate in clinical trials in cancer settings: structural (eg, availability of a clinical trial), clinical (ie, eligibility to the clinical trial), attitudinal (ie, patients’ and clinicians’ attitudes), and demographic and socioeconomic (eg, age, sex, minorities, income).65 Inclusion/exclusion criteria may have been too strict, and thus may have reduced the total number of eligible patients in those studies as well as in the present one. The lack of interest of the cancer patients was identified as an important barrier to the participation in clinical trials.65 Indeed, 57% of the eligible cancer patients were not interested in participating in studies evaluating MI to increase PA. However, this percentage is close to the percentage observed for other noncommunicable diseases and cannot explain the lower recruitment rate observed in cancer settings. In the present study, most of the patients were recruited via the treating oncologists (92%). Nevertheless, one single oncologist recruited 76% of the participants. This oncologist is strongly engaged in PA promotion as she is the president of an association offering PA for cancer patients. The limited involvement of the other clinicians might have acted as a significant barrier to a more successful recruitment. In the literature, several factors were observed to explain the low involvement of the clinicians. These factors include, among others, the nature of the study regimen, interferences in the clinician-patient relationship, lack of incentives, and lack of time.65 In addition, the specific context of PA counseling may also contribute to the limited involvement of the clinicians. Indeed, PA is not often recommended to the patients during their medical consultation.24 For example, only 24% to 29% of the patients received PA counseling/encouragement from their general practitioners in Luxembourg.66 Structural changes (eg, time paid for research and/or prevention counseling, training to promote PA) might result in a better involvement of medical doctors. The efforts undertaken within the current study to encourage the active involvement of the oncologists may have been insufficient.
Table 2.
Study Design, Recruitment, and MI Delivery of the Known Studies That Used MI to Increase PA Level in Cancer Patients.
| First Author, Year | Study Design | Recruitment Period | Screened Patients, n | Eligible Patients, n (%) | Included Patients, n (%) | Attrition, n (%) | MI Duration | MI Delivery |
|---|---|---|---|---|---|---|---|---|
| Swenson, 2010 | Cohort | 32 months | — | — | E: 36 | E: 7 (19%) | 12 months | E: in-person for at least 4 chemotherapy cycles |
| Garrett, 2013 | Cohort | — | — | — | E: 66 | E: 20 (30%) | 3 months | E: 6 phone calls |
| Spector, 2014 | Cohort | — | — | — | E: 17 | E: 4 (23%) | 4 months | E: 1 in-person, 15 phone calls |
| Braun, 2018 | Nonrandomized clinical trial | — | — | — | T: 29 | — | 6 months | E: 4 tele-MI sessions |
| E: 17 | ||||||||
| C: 12 | C: usual care | |||||||
| Bennett, 2007 | RCT | 6 months | 179 | 84 (47%) | T: 56 (58%) | E: 7 (25%) | 6 months | E: 3 in-person, 3 phone calls |
| E: 28 | ||||||||
| C: 28 | C: 2 (7%) | C: 3 in-person, 3 phone calls without MI | ||||||
| Djuric, 2011 | RCT | 10 months | 77 | 72 (93%) | T: 40 (56%) | E: 7 (35%) | 12 months | E: 19 phone calls |
| E: 20 | ||||||||
| C: 20 | C: 3 (15%) | C: information and pedometer | ||||||
| Asvat Patel, 2013 | RCT | — | 513 | 269 (52%) | T: 66 (24%) | E: 8 (24%) | 5 weeks | E: 2 in-person, one phone call |
| E: 33 | ||||||||
| C: 33 | C: 7 (21%) | C: usual care | ||||||
| Sheppard, 2016 | RCT | 14 months | 106 | 69 (65%) | T: 31 (45%) | E: 5 (33%) | 3 months | E: 6 group session, 6 phone calls |
| E: 15 | ||||||||
| C: 16 | C: 4 (25%) | C: usual care | ||||||
| Dennett, 2018 | RCT | 13 months | 80 | 57 (71%) | T: 46 (81%) | E: 3 (13%) | 7 weeks | E: 7 phone calls |
| E: 22 | ||||||||
| C: 24 | C: 1 (4%) | C: usual care | ||||||
| Current study | RCT | 16 months | — | — | T: 25 | E: 5 (38%) | 3 months | E: 2 in-person, 10 phone calls |
| E: 13 | ||||||||
| C: 12 | C: 1 (8%) | C: usual care |
Abbreviations: MI, motivational interviewing; PA, physical activity; RCT, randomized controlled trial; T, total participants; E, experimental group; C, control group.
In the present study, 5 (38.5%) of the 13 participants from the experimental group dropped out during the intervention, whereas only one participant (8.3%) left the control group in the same period of time. The dropout participants were older, and fewer had radiotherapy as well as chemotherapy treatment (nonsignificant difference). We might speculate that those participants needing less treatment might feel less threatened by their condition, and thus be less inclined to change their lifestyle. The attrition rate during the intervention observed in similar RCTs for cancer patients was 25% (from 14% to 35%) in the experimental groups and 14% (from 4% to 25%) in the control groups (Table 2).44-48 As in the present investigation, attrition rates in those studies were consistently higher in the experimental groups than in the control groups. Medical and unknown reasons were mostly cited to explain these attrition rates.44-48 For comparison, the attrition rate at the end of the intervention observed in RCTs for patients with other noncommunicable diseases was 29% (from 0% to 54%) in the experimental groups and 28% (from 0% to 50%) in the control groups (n = 23 studies).34-37 The reported reasons of the attrition were mainly related to the diseases.
In the present study, the duration of the MI sessions was similar for the patients who completed the intervention than for those who dropped out. Moreover, the average duration did not differ from the duration (20-25 minutes for the phone calls) observed in similar RCTs with cancer patients.44,47 Obviously, the number of completed MI sessions was 3 times lower in participants who dropped out. The higher attrition rate in the experimental group could thus be due to participants’ (unmet) expectations regarding the MI intervention. Indeed, some participants thought that the MI intervention was a sort of coaching with a defined and precise PA program. To avoid misinterpretations from the participants, objectives and modalities of the intervention should be more clearly explained by the oncologists and researchers. Moreover, several MI appointments were missed, postponed, or refused during the intervention phase, which suggests that participants experienced the MI sessions as time consuming and sometimes disruptive. The relative lack of experience of the counselors might also explain this adherence issue. Nevertheless, the percentage of MI sessions (86%) completed by those who did not drop out was in the same range than those reported by similar RCTs (from 86% to 98%).44,47,48
Taking the attrition rate and the percentage of completed MI sessions together, we would argue that in the current study, the MI intervention was moderately accepted by participants.
Potential Efficacy of MI Intervention
MI intervention was proposed to the participants of the experimental group to increase their level of MVPA and physical fitness, as well as to improve quality of life. However, due to the small sample size of the present pilot study, any conclusion about the potential efficacy of the MI intervention with regard to these variables would be highly speculative. In a literature review, including 3 cohort studies and 3 RCTs (1 RCT evaluated the effect of the intervention via body weight changes67), Spencer and Wheeler41 concluded that MI leads to a moderate but significant improvement of PA behavior in cancer patients. In addition to the 2 RCTs from this review, which used PA behavior as a study outcome,47,48 3 more RCTs were identified.44-46 However, none of these 5 RCTs observed differences in PA behavior between the experimental and control groups at the end of the MI intervention. Because of their small sample sizes, high attrition rates, and various study designs (eg, frequency and type of MI sessions, measurement of the PA, etc), no conclusion can be drawn from these RCTs regarding the efficacy of MI to improve PA in cancer patients.
Limitations of the Study
In addition to the small sample size, the present study has other limitations. First, the inclusion of an attention control group would have allowed for any effects specific to the MI to be more clearly delineated from more unspecific effects of social contact. Second, the contact time with the counselor should have been the same for the control and the intervention group. This would have helped eliminate the possible confounding effect of differences in contact time with the counselor. Nevertheless, a recent systematic review of reviews of the effectiveness of MI intervention shows consistent evidence that MI is beneficial (whatever the targeted health issue) when compared with “weak” comparison groups.68 In the present study, participants were recruited whether they were physically active or not. However, the possible effect of the MI intervention would probably be maximized in the patients who are least active and who want to change their PA behavior. To better assess the feasibility of the intervention, 8 areas of focus should have been investigated: acceptability, demand, implementation, practicality, adaptation, integration, expansion, and limited-efficacy testing.52 However, it seems important to perform the study in the most ecological approach, that is, limiting additional investigations that could also hamper the study. The results of the present study should be interpreted with regard to these limitations.
Considerations for Future RCTs and MI Implementation in Cancer Care
The present study and the scientific literature cannot lead to definite conclusions on the effectiveness of MI to increase PA in cancer patients. Therefore, further RCTs are needed. Based on the outcomes of the present pilot study, as well as other previous studies, we recommend overrecruitment of 30% to 40% to accommodate for the elevated observed attrition rate. Moreover, the perception and attitudes of oncologists and clinicians may be critical to encourage their patients to participate in such a study. RCTs should target the patients who are the most in need, such as patients with low PA level, low quality of life, low physical function, and fatigue.29 The duration of the intervention delivery should be sufficiently long, and the frequency of the MI should be high.68 We also think that experience of the counselors may be critical for MI interventions to avoid high dropout rates. Beside these scientific considerations, and if MI interventions are proven efficient in the context of cancer survivors, major barriers for their implementation seem to be lack of interest to enter such a program and to adhere to the intervention. Finally, the cost-effectiveness of the intervention should also be investigated in further RCTs as it represents an important parameter for policymakers.
Conclusion
Increasing PA levels is known to confer health benefits in cancer patients. The assurance of a long-term PA behavior change represents, however, a big challenge. MI intervention appeared to be an interesting solution to tackle this problem. However, the small sample size and the high attrition rate in the experimental group observed in the present study indicate a low feasibility and acceptability of our protocol. Therefore, no conclusion should be drawn regarding the potential efficacy of a MI intervention at this stage. A larger RCT with an optimized recruitment, communication strategy, and study design is needed to reliably investigate whether it is worth implementing a MI intervention in cancer care in Luxembourg.
Supplemental Material
Supplemental material, ICT_-_Supplementary_Material_1_-_revised_version_-_V2 for Motivational Interviewing to Increase Physical Activity Behavior in Cancer Patients: A Pilot Randomized Controlled Trials by Alexis Lion, Anne Backes, Caroline Duhem, Fernand Ries, Charles Delagardelle, Axel Urhausen, Claus Vögele, Daniel Theisen and Laurent Malisoux in Integrative Cancer Therapies
Supplemental material, ICT_-_Supplementary_Material_2_-_revised_version_-_V2 for Motivational Interviewing to Increase Physical Activity Behavior in Cancer Patients: A Pilot Randomized Controlled Trials by Alexis Lion, Anne Backes, Caroline Duhem, Fernand Ries, Charles Delagardelle, Axel Urhausen, Claus Vögele, Daniel Theisen and Laurent Malisoux in Integrative Cancer Therapies
Acknowledgments
The authors would like to thank the Fondation Cancer, which provided financial support for the study. The authors would also like to thank the following persons involved in this study: Dr Patrick Feiereisen (Centre Hospitalier de Luxembourg) for his assistance in the design of the study; Ms Hélène Agostinis and Ms Jessica Calmes (both from the Luxembourg Institute of Health) who conducted the MI; Mr Eric Besenius (Luxembourg Institute of Health) for the assistance in the data acquisition and analyses; Dr Susanne Schmitz and Ms Anna Schritz (both from the Luxembourg Institute of Health) for the assistance in the statistical analyses; and Dr Claude Besenius (Centre Hospitalier Neuro-Psychiatrique) for the evaluation of the MI intervention. The authors would like to thank the oncologists, radiotherapists, and surgeons from the main hospitals in Luxembourg for their help in the recruitment process. AL is grateful to AMP and RL for their support.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Fondation Cancer (Grant Number FC/2017/02).
ORCID iDs: Alexis Lion  https://orcid.org/0000-0002-3807-5177
https://orcid.org/0000-0002-3807-5177
Laurent Malisoux  https://orcid.org/0000-0002-6601-5630
https://orcid.org/0000-0002-6601-5630
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Courneya KS, Segal RJ, McKenzie DC, et al. Effects of exercise during adjuvant chemotherapy on breast cancer outcomes. Med Sci Sports Exerc. 2014;46:1744-1751. doi: 10.1249/MSS.0000000000000297 [DOI] [PubMed] [Google Scholar]
- 2. Meyerhardt JA, Heseltine D, Niedzwiecki D, et al. Impact of physical activity on cancer recurrence and survival in patients with stage III colon cancer: findings from CALGB 89803. J Clin Oncol. 2006;24:3535-3541. doi: 10.1200/JCO.2006.06.0863 [DOI] [PubMed] [Google Scholar]
- 3. Lahart IM, Metsios GS, Nevill AM, Carmichael AR. Physical activity, risk of death and recurrence in breast cancer survivors: a systematic review and meta-analysis of epidemiological studies. Acta Oncol. 2015;54:635-654. doi: 10.3109/0284186X.2014.998275 [DOI] [PubMed] [Google Scholar]
- 4. McTiernan A, Friedenreich CM, Katzmarzyk PT, et al. ; 2018 Physical Activity Guidelines Advisory Committee. Physical activity in cancer prevention and survival. Med Sci Sport Exerc. 2019;51:1252-1261. doi: 10.1249/MSS.0000000000001937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Patel AV, Friedenreich CM, Moore SC, et al. American College of Sports Medicine Roundtable report on physical activity, sedentary behavior, and cancer prevention and control. Med Sci Sports Exerc. 2019;51:2391-2402. doi: 10.1249/MSS.0000000000002117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fairman CM, Focht BC, Lucas AR, Lustberg MB. Effects of exercise interventions during different treatments in breast cancer. J Community Support Oncol. 2016;14:200-209. doi: 10.12788/jcso.0225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kalter J, Kampshoff CS, Chinapaw MJM, et al. Mediators of exercise effects on HRQoL in cancer survivors after chemotherapy. Med Sci Sports Exerc. 2016;48:1859-1865. doi: 10.1249/MSS.0000000000000976 [DOI] [PubMed] [Google Scholar]
- 8. Sellar CM, Bell GJ, Haennel RG, Au HJ, Chua N, Courneya KS. Feasibility and efficacy of a 12-week supervised exercise intervention for colorectal cancer survivors. Appl Physiol Nutr Metab. 2014;39:715-723. doi: 10.1139/apnm-2013-0367 [DOI] [PubMed] [Google Scholar]
- 9. Gilchrist SC, Barac A, Ades PA, et al. Cardio-oncology rehabilitation to manage cardiovascular outcomes in cancer patients and survivors: a scientific statement from the American Heart Association. Circulation. 2019;139:e997-e1012. doi: 10.1161/CIR.0000000000000679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dennett AM, Peiris CL, Shields N, Prendergast LA, Taylor NF. Moderate-intensity exercise reduces fatigue and improves mobility in cancer survivors: a systematic review and meta-regression. J Physiother. 2016;62:68-82. doi: 10.1016/j.jphys.2016.02.012 [DOI] [PubMed] [Google Scholar]
- 11. Mishra SI, Scherer RW, Geigle PM, et al. Exercise interventions on health-related quality of life for cancer survivors. Cochrane Database Syst Rev. 2012;(8):CD007566. doi: 10.1002/14651858.CD007566.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McMillan EM, Newhouse IJ. Exercise is an effective treatment modality for reducing cancer-related fatigue and improving physical capacity in cancer patients and survivors: a meta-analysis. Appl Physiol Nutr Metab. 2011;36:892-903. doi: 10.1139/H11-082 [DOI] [PubMed] [Google Scholar]
- 13. Park JH, Lee J, Oh M, et al. The effect of oncologists’ exercise recommendations on the level of exercise and quality of life in survivors of breast and colorectal cancer: a randomized controlled trial. Cancer. 2015;121:2740-2748. doi: 10.1002/cncr.29400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Campbell KL, Winters-Stone KM, Wiskemann J, et al. Exercise guidelines for cancer survivors: consensus statement from International Multidisciplinary Roundtable. Med Sci Sports Exerc. 2019;51:2375-2390. doi: 10.1249/MSS.0000000000002116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Irwin ML, McTiernan A, Bernstein L, Gilliland FD, Baumgartner R, Baumgartner K. Physical activity levels among breast cancer survivors. Med Sci Sport Exerc. 2004;36:1484-1491. [PMC free article] [PubMed] [Google Scholar]
- 16. Mason C, Alfano CM, Smith AW, et al. Long-term physical activity trends in breast cancer survivors. Cancer Epidemiol Biomarkers Prev. 2013;22:1153-1161. doi: 10.1158/1055-9965.EPI-13-0141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Arem H, Pfeiffer RM, Moore SC, Brinton LA, Matthews CE. Body mass index, physical activity, and television time in relation to mortality risk among endometrial cancer survivors in the NIH-AARP Diet and Health Study cohort. Cancer Causes Control. 2016;27:1403-1409. doi: 10.1007/s10552-016-0813-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Troeschel AN, Leach CR, Shuval K, Stein KD, Patel AV. Physical activity in cancer survivors during “re-entry” following cancer treatment. Prev Chronic Dis. 2018;15:E65. doi: 10.5888/pcd15.170277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Basen-Engquist K, Scruggs S, Jhingran A, et al. Physical activity and obesity in endometrial cancer survivors: associations with pain, fatigue, and physical functioning. Am J Obstet Gynecol. 2009;200:288.e1-288.e8. doi: 10.1016/j.ajog.2008.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Barker J, Smith Byrne K, Doherty A, et al. Physical activity of UK adults with chronic disease: cross-sectional analysis of accelerometer-measured physical activity in 96 706 UK biobank participants. Int J Epidemiol. 2019;48:1167-1174. doi: 10.1093/ije/dyy294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Huy C, Schmidt ME, Vrieling A, Chang-Claude J, Steindorf K. Physical activity in a German breast cancer patient cohort: one-year trends and characteristics associated with change in activity level. Eur J Cancer. 2012;48:297-304. doi: 10.1016/j.ejca.2011.08.005 [DOI] [PubMed] [Google Scholar]
- 22. Nelson SH, Weiner LS, Natarajan L, Parker BA, Patterson RE, Hartman SJ. Continuous, objective measurement of physical activity during chemotherapy for breast cancer: the activity in treatment pilot study [published online May 29, 2019]. Transl Behav Med. doi: 10.1093/tbm/ibz079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Browall M, Mijwel S, Rundqvist H, Wengström Y. Physical activity during and after adjuvant treatment for breast cancer: an integrative review of women’s experiences. Integr Cancer Ther. 2018;17:16-30. doi: 10.1177/1534735416683807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lion A, Vuillemin A, Thornton JS, Theisen D, Stranges S, Ward M. Physical activity promotion in primary care: a Utopian quest? Health Promot Int. 2019;34:877-886. doi: 10.1093/heapro/day038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schmitz KH, Campbell AM, Stuiver MM, et al. Exercise is medicine in oncology: engaging clinicians to help patients move through cancer. CA Cancer J Clin. 2019;69:468-484. doi: 10.3322/caac.21579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nyrop KA, Deal AM, Williams GR, Guerard EJ, Pergolotti M, Muss HB. Physical activity communication between oncology providers and patients with early-stage breast, colon, or prostate cancer. Cancer. 2016;122:470-476. doi: 10.1002/cncr.29786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Segar ML, Guérin E, Phillips E, Fortier M. From a vital sign to vitality: selling exercise so patients want to buy it. Curr Sports Med Rep. 2016;15:276-281. doi: 10.1249/JSR.0000000000000284 [DOI] [PubMed] [Google Scholar]
- 28. Pinto BM, Floyd A. Theories underlying health promotion interventions among cancer survivors. Semin Oncol Nurs. 2008;24:153-163. doi: 10.1016/j.soncn.2008.05.003 [DOI] [PubMed] [Google Scholar]
- 29. Grimmett C, Corbett T, Brunet J, et al. Systematic review and meta-analysis of maintenance of physical activity behaviour change in cancer survivors. Int J Behav Nutr Phys Act. 2019;16:37. doi: 10.1186/s12966-019-0787-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rollnick S, Miller WR. What is motivational interviewing? Behav Cogn Psychother. 1995;23:325-334. doi: 10.1017/S135246580001643X [DOI] [PubMed] [Google Scholar]
- 31. Lundahl BW, Kunz C, Brownell C, Tollefson D, Burke BL. A meta-analysis of motivational interviewing: twenty-five years of empirical studies. Res Soc Work Pract. 2010;20:137-160. doi: 10.1177/1049731509347850 [DOI] [Google Scholar]
- 32. Lundahl BW, Burke BL. The effectiveness and applicability of motivational interviewing: a practice-friendly review of four meta-analyses. J Clin Psychol. 2009;65:1232-1245. doi: 10.1002/jclp.20638 [DOI] [PubMed] [Google Scholar]
- 33. Martins RK, McNeil DW. Review of motivational interviewing in promoting health behaviors. Clin Psychol Rev. 2009;29:283-293. doi: 10.1016/j.cpr.2009.02.001 [DOI] [PubMed] [Google Scholar]
- 34. O’Halloran PD, Blackstock F, Shields N, et al. Motivational interviewing to increase physical activity in people with chronic health conditions: a systematic review and meta-analysis. Clin Rehabil. 2014;28:1159-1171. doi: 10.1177/0269215514536210 [DOI] [PubMed] [Google Scholar]
- 35. Poudel N, Kavookjian J, Scalese MJ. Motivational interviewing as a strategy to impact outcomes in heart failure patients: a systematic review. Patient. 2020;13:43-55. doi: 10.1007/s40271-019-00387-6 [DOI] [PubMed] [Google Scholar]
- 36. Soderlund PD. Effectiveness of motivational interviewing for improving physical activity self-management for adults with type 2 diabetes: a review. Chronic Illn. 2018;14:54-68. doi: 10.1177/1742395317699449 [DOI] [PubMed] [Google Scholar]
- 37. Barrett S, Begg S, O’Halloran P, Kingsley M. Integrated motivational interviewing and cognitive behaviour therapy for lifestyle mediators of overweight and obesity in community-dwelling adults: a systematic review and meta-analyses. BMC Public Health. 2018;18:1160. doi: 10.1186/s12889-018-6062-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gustavsson C, Nordqvist M, Bröms K, Jerdén L, Kallings LV, Wallin L. What is required to facilitate implementation of Swedish physical activity on prescription? Interview study with primary healthcare staff and management. BMC Health Serv Res. 2018;18:196. doi: 10.1186/s12913-018-3021-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. NICE. Physical activity: exercise referral schemes. https://www.nice.org.uk/guidance/ph54. Published September 2014. Accessed March 7, 2020.
- 40. Haute Autorité de Santé. Promotion, consultation et prescription médicale d’activité physique et sportive pour la santé. https://www.has-sante.fr/portail/jcms/c_2876862/fr/promotion-consultation-et-prescription-medicale-d-activite-physique-et-sportive-pour-la-sante. Published October 17, 2018. Accessed March 7, 2020.
- 41. Spencer JC, Wheeler SB. A systematic review of motivational interviewing interventions in cancer patients and survivors. Patient Educ Couns. 2016;99:1099-1105. doi: 10.1016/j.pec.2016.02.003 [DOI] [PubMed] [Google Scholar]
- 42. Pudkasam S, Polman R, Pitcher M, et al. Physical activity and breast cancer survivors: Importance of adherence, motivational interviewing and psychological health. Maturitas. 2018;116:66-72. doi: 10.1016/j.maturitas.2018.07.010 [DOI] [PubMed] [Google Scholar]
- 43. Braun A, Portner J, Grainger EM, et al. Tele-motivational interviewing for cancer survivors: feasibility, preliminary efficacy, and lessons learned. J Nutr Educ Behav. 2018;50:19-32.e1. doi: 10.1016/j.jneb.2017.05.352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Dennett AM, Shields N, Peiris CL, et al. Motivational interviewing added to oncology rehabilitation did not improve moderate-intensity physical activity in cancer survivors: a randomised trial. J Physiother. 2018;64:255-263. doi: 10.1016/j.jphys.2018.08.003 [DOI] [PubMed] [Google Scholar]
- 45. Asvat Patel Y. Motivational Interviewing to Promote Physical Activity in Breast Cancer Survivors [dissertation]. Tampa, FL: University of South Florida; 2013. http://scholarcommons.usf.edu/etd/4629. Accessed April 30, 2019. [Google Scholar]
- 46. Sheppard VB, Hicks J, Makambi K, Hurtado-de-Mendoza A, Demark-Wahnefried W, Adams-Campbell L. The feasibility and acceptability of a diet and exercise trial in overweight and obese black breast cancer survivors: the Stepping STONE study. Contemp Clin Trials. 2016;46:106-113. doi: 10.1016/j.cct.2015.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bennett JA, Lyons KS, Winters-Stone K, Nail LM, Scherer J. Motivational interviewing to increase physical activity in long-term cancer survivors: a randomized controlled trial. Nurs Res. 2007;56:18-27. doi: 10.1097/00006199-200701000-00003 [DOI] [PubMed] [Google Scholar]
- 48. Djuric Z, Ellsworth JS, Weldon AL, et al. A diet and exercise intervention during chemotherapy for breast cancer. Open Obes J. 2011;3:87-97. doi: 10.2174/1876823701103010087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Spector D, Deal AM, Amos KD, Yang H, Battaglini CL. A pilot study of a home-based motivational exercise program for African American breast cancer survivors: clinical and quality-of-life outcomes. Integr Cancer Ther. 2014;13:121-132. doi: 10.1177/1534735413503546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Swenson KK, Nissen MJ, Henly SJ. Physical activity in women receiving chemotherapy for breast cancer: adherence to a walking intervention. Oncol Nurs Forum. 2010;37:321-330. doi: 10.1188/10.ONF.321-330 [DOI] [PubMed] [Google Scholar]
- 51. Garrett K, Okuyama S, Jones W, et al. Bridging the transition from cancer patient to survivor: Pilot study results of the Cancer Survivor Telephone Education and Personal Support (C-STEPS) program. Patient Educ Couns. 2013;92:266-272. doi: 10.1016/j.pec.2013.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bowen DJ, Kreuter M, Spring B, et al. How we design feasibility studies. Am J Prev Med. 2009;36:452-457. doi: 10.1016/j.amepre.2009.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Vähä-Ypyä H, Vasankari T, Husu P, et al. Validation of cut-points for evaluating the intensity of physical activity with accelerometry-based mean amplitude deviation (MAD). PLoS One. 2015;10:e0134813. doi: 10.1371/journal.pone.0134813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Leinonen AM, Ahola R, Kulmala J, et al. Measuring physical activity in free-living conditions: Comparison of three accelerometry-based methods. Front Physiol. 2017;7:681. doi: 10.3389/fphys.2016.00681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Vähä-Ypyä H, Vasankari T, Husu P, Suni J, Sievänen H. A universal, accurate intensity-based classification of different physical activities using raw data of accelerometer. Clin Physiol Funct Imaging. 2015;35:64-70. doi: 10.1111/cpf.12127 [DOI] [PubMed] [Google Scholar]
- 56. Vähä-Ypyä H, Husu P, Suni J, Vasankari T, Sievänen H. Reliable recognition of lying, sitting, and standing with a hip-worn accelerometer. Scand J Med Sci Sport. 2018;28:1092-1102. doi: 10.1111/sms.13017 [DOI] [PubMed] [Google Scholar]
- 57. Loyen A, Clarke-Cornwell AM, Anderssen SA, et al. Sedentary time and physical activity surveillance through accelerometer pooling in four European countries. Sport Med. 2017;47:1421-1435. doi: 10.1007/s40279-016-0658-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Jones CJ, Rikli RE, Beam WC. A 30-s chair-stand test as a measure of lower body strength in community-residing older adults. Res Q Exerc Sport. 1999;70:113-119. doi: 10.1080/02701367.1999.10608028 [DOI] [PubMed] [Google Scholar]
- 59. Roberts HC, Denison HJ, Martin HJ, et al. A review of the measurement of grip strength in clinical and epidemiological studies: towards a standardised approach. Age Ageing. 2011;40:423-429. doi: 10.1093/ageing/afr051 [DOI] [PubMed] [Google Scholar]
- 60. Will PM, Walter JD. Exercise testing: improving performance with a ramped Bruce protocol. Am Heart J. 1999;138(6 pt 1):1033-1037. [DOI] [PubMed] [Google Scholar]
- 61. Tanaka H, Monahan KD, Seals DR. Age-predicted maximal heart rate revisited. J Am Coll Cardiol. 2001;37:153-156. [DOI] [PubMed] [Google Scholar]
- 62. Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res. 2011;20:1727-1736. doi: 10.1007/s11136-011-9903-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Viechtbauer W, Smits L, Kotz D, et al. A simple formula for the calculation of sample size in pilot studies. J Clin Epidemiol. 2015;68:1375-1379. doi: 10.1016/j.jclinepi.2015.04.014 [DOI] [PubMed] [Google Scholar]
- 64. Hammer G, Golinska B, Mittelbronn M. Nouveaux Cas de Cancer Au Grand-Duché de Luxembourg Diagnostiqués Au LNS—Années 2014-2017. Registre Morphologique Des Tumeurs; 2018. [Google Scholar]
- 65. Unger JM, Cook E, Tai E, Bleyer A. The role of clinical trial participation in cancer research: barriers, evidence, and strategies. Am Soc Clin Oncol Educ B. 2016;36:185-198. doi: 10.14694/edbk_156686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Lion A, Lethal J, Urhausen A, Theisen D, Seil R, Delagardelle C. Effectiveness of a national campaign promoting physical activity for patients with non-communicable diseases in Luxembourg. Paper presented at: 10th HEPA Europe Conference; Odense, Denmark; 2019. [Google Scholar]
- 67. Harris MN, Swift DL, Myers VH, et al. Cancer survival through lifestyle change (CASTLE): a pilot study of weight Loss. Int J Behav Med. 2013;20:403-412. doi: 10.1007/s12529-012-9234-5 [DOI] [PubMed] [Google Scholar]
- 68. Frost H, Campbell P, Maxwell M, et al. Effectiveness of motivational interviewing on adult behaviour change in health and social care settings: a systematic review of reviews. PLoS One. 2018;13:e0204890. doi: 10.1371/journal.pone.0204890 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, ICT_-_Supplementary_Material_1_-_revised_version_-_V2 for Motivational Interviewing to Increase Physical Activity Behavior in Cancer Patients: A Pilot Randomized Controlled Trials by Alexis Lion, Anne Backes, Caroline Duhem, Fernand Ries, Charles Delagardelle, Axel Urhausen, Claus Vögele, Daniel Theisen and Laurent Malisoux in Integrative Cancer Therapies
Supplemental material, ICT_-_Supplementary_Material_2_-_revised_version_-_V2 for Motivational Interviewing to Increase Physical Activity Behavior in Cancer Patients: A Pilot Randomized Controlled Trials by Alexis Lion, Anne Backes, Caroline Duhem, Fernand Ries, Charles Delagardelle, Axel Urhausen, Claus Vögele, Daniel Theisen and Laurent Malisoux in Integrative Cancer Therapies