Abstract
Background
Transplantation of the liver entails a state of altered recipient immunologic competence. There are only scarce data concerning the impact of host immunologic factors on the outcome of liver transplant recipients in the context of hepatocellular carcinoma (HCC).
Material/Methods
Our study focused on evaluating the presence of tumor necrosis and frequency levels of angiopoietins and monocytes/macrophages subtypes in the host liver prior to liver transplantation (LTX) and their association with recurrence, graft rejection, survival, and clinical prognosis after LTX. Formation of tumor necrosis and tissue densities of angiopoietins and cellular immunologic infiltrates – CD68+ and CD163+ macrophages (TAMs) and TIE2-expressing monocytes (TEMs) – were quantified in recipient HCC specimens. The densities were then matched with clinicopathologic variables and patient survival after LTX (n=88). Some patients were treated prior to LTX by neoadjuvant transarterial chemoembolization (TACE, n=55).
Results
Recipient hepatic infiltration with TEMs and CD68+ TAMs was associated with decreased 1-, 3-, and 5-year survival, as well as metastatic and recurrent HCC after LTX (all p<0.05). TEMs and infiltrating monocytes/macrophages were associated with angiopoietin expression, metastatic, and recurrent HCC (all p<0.05). Furthermore, hepatic angiopoietin-2 expression was associated with graft rejection after LTX (p<0.05). After TACE and LTX, formation of tumor necrosis was associated with an increased presence of monocytes/macrophages and a reduced incidence of recurrent HCC in the graft (all p<0.05).
Conclusions
Infiltrating monocytes/macrophages subsets and related angiopoietin axis are associated with worse survival, tumor recurrence, and clinical outcome after LTX for HCC.
MeSH Keywords: Angiopoietins; Carcinoma, Hepatocellular; Liver Transplantation; Monocytes
Background
Hepatocellular carcinoma (HCC) is a common frequent human malignancy and contributes significantly to worldwide cancer-related morbidity and mortality [1]. Moreover, the incidence of HCC in Asia and Western countries, including the USA, has grown markedly in recent years, and, according to estimates, it will continuously rise in the next 2 decades [1]. In cases of unresectable HCC, LTX is a standard therapeutic modality that offers a realistic chance of healing. Many HCC transplant candidates receive a bridging therapy – transarterial chemoembolization (TACE) – prior to LTX [2].
Chronic hepatic inflammation and autoimmune disorders that progress to liver cirrhosis are key mediators in hepatocarcinogenesis [3]. However, the mechanisms by which chronic inflammation influences this process are not well understood. Furthermore, it is unclear to what extent these tumor infiltrates of immune-competent cells in the recipient liver prior to LTX affect disease outcome after transplantation.
Tumor-associated macrophages (TAMs) are essential mediators of inflammation in the vicinity of cancer [4]. They contribute to tumor progression by deploying many different mechanisms comprising genetic instability, nurturing cancer stem cells, enhancing the process of metastasis, and reducing the potency of the protective adaptive immunity [5]. Depending on the established macrophage polarization state (M1 or M2 functional mode) in the tumor microenvironment, TAMs can profoundly influence the effectiveness of cytoreductive chemotherapies, either by antagonizing immunologic anti-tumor activity or contributing to the antineoplastic efficacy [6]. Furthermore, monocyte/macrophage functions in the tumor microenvironment have been mechanistically linked to the formation of tumor necrosis [7]. The occurrence of treatment-induced histologic necrosis in cancerous tissues after neoadjuvant therapies has also been associated with tumor recurrence and patient outcomes [8].
Neoangiogenesis in the highly vascularized tumor microenvironment of HCC is an established modulator of hepatocarcinogenesis and is an attractive opportunity for anticancer therapy [9]. In this setting, the TIE2-angiopoietin system exerts a significant impact on tumor progression and has been recognized as an essential anti-angiogenic pathway for future drug development [10]. Angiopoietins, especially angiopoietin-1 and -2, have recently been shown to be expressed in tumorous tissues, such as hepatocellular carcinoma [11]. The angiopoietin family comprises several factors of angiogenesis that interact with the TIE2 receptor and regulate the pathological and physiological angiogenic processes [12]. CD14+ non-classical monocytes expressing the functional angiopoietin TIE2 receptor contribute significantly to neovascularization processes in solid tumors, utilizing paracrine mechanisms in the vicinity of tumorous neo-vessels [12]. These TIE-expressing monocytes (TEMs) are M2 phenotyped and are responsible for most of the angiogenic activity in the tumor microenvironment [13].
The immunologic competence of the recipient is profoundly altered after solid organ transplantation. In this setting, the clinical significance of recipient-related factors of angiogenesis, related tumor infiltrating monocytes/macrophages, and formation of necrosis in the tumor microenvironment concerning tumor progression is unknown. Thus, based on previous results on the importance of the immune system in hepatobiliary cancer, we hypothesized that, in the recipient’s native tumor microenvironment prior transplantation, a coherent construct composed of infiltrating monocytes/macrophages, related necrosis, and factors of angiogenesis exert a significant effect on clinical outcome after LTX. In the present study, we demonstrate that, in the microenvironment of HCC, the tissue densities of the above-mentioned biomarkers are strongly associated. Furthermore, we showed that they have a substantial influence on graft rejection, incidence of metastatic disease, and survival of patients after transplantation.
Material and Methods
Patients and tumor samples
The Institutional Ethics Commission approved this retrospective study (no. 234-14-14072014). Our study included 88 liver transplant recipients with HCC arising de novo in cirrhotic livers. The study period began on 10 April 2002 and ended on 10 April 2015. Inclusion criteria comprised patients with histologically confirmed HCC with no chemotherapy or radiation prior surgery who received LTX. No pediatric patients were included and none of the patients received antiangiogenic treatment modalities (e.g., sorafenib or prior LTX). LTX patients who received re-transplant in the further course or who died within 90 days after the surgery were excluded from the study. The median waiting time for LTX was 5.05 months for patients who received bridging therapy to LTX with TACE and 5.4 months for patients without TACE. Patients with ‘high urgency’ status had a median waiting time for LTX of 2 days. The clinical background, underlying liver disease, and demographics of the patients are summarized in Table 1. Figure 1 depicts a flowchart describing the patient selection process for our study.
Table 1.
Clinicopathological characteristics of patients undergoing liver transplantation for hepatocellular carcinoma.
| Variable | Without TACE (n=33) | With TACE (n=55) | p |
|---|---|---|---|
| Patient age (years) | |||
| ≤60 | 14 (42.4) | 23 (41.8) | 0.956 |
| >60 | 19 (57.6) | 32 (58.2) | |
| Gender | |||
| Male | 26 (78.8) | 47 (85.5) | 0.421 |
| Female | 7 (21.2) | 8 (14.5) | |
| Graft tumour recurrence | |||
| With | 5 (15.2) | 6 (10.9) | 0.560 |
| Without | 28 (84.8) | 49 (89.1) | |
| Distant metastases | |||
| With | 4 (12.1) | 9 (16.4) | 0.007 |
| Without | 29 (87.9) | 46 (83.6) | |
| Graft rejection | |||
| With | 16 (48.5) | 13 (23.6) | 0.016 |
| 6Without | 17 (51.5) | 42 (76.4) | |
| Tumour size (mm) | |||
| ≤20 | 19 (57.6) | 44 (80.0) | 0.030 |
| >20 | 14 (42.4) | 11 (20.0) | |
| Angioinvasion | |||
| Positive | 4 (12.1) | 6 (10.9) | 0.862 |
| Negative | 29 (87.9) | 49 (89.1) | |
| Lymphangiosis carcinomatosa | |||
| Positive | 1 (3.0) | 7 (12.7) | 0.126 |
| Negative | 32 (97.0) | 48 (87.3) | |
| Histologic differentiation | |||
| G1 well | 15 (45.5) | 29 (52.7) | 0.509 |
| G2 moderate/G3 poor | 18 (54.5) | 26 (47.3) | |
| Pathologic T stage | |||
| T1 | 28 (84.8) | 27 (49.1) | 0.001 |
| T2/T3 | 5 (15.2) | 28 (50.9) | |
| Alcoholic liver cirrhosis | |||
| With | 19 (57.6) | 40 (72.7) | 0.143 |
| Without | 14 (42.4) | 15 (27.3) | |
| Viral hepatitis | |||
| With | 10 (30.3) | 9 (16.4) | 0.124 |
| Without | 23 (69.7) | 46 (83.6) | |
| NASH | |||
| With | 2 (6.1) | 7 (12.7) | 0.318 |
| Without | 31 (93.9) | 48 (87.3) | |
| Hemochromatosis | |||
| With | 2 (6.1) | 0 (0.0) | 0.065 |
| Without | 31 (93.9) | 55 (100.0) | |
Figure 1.
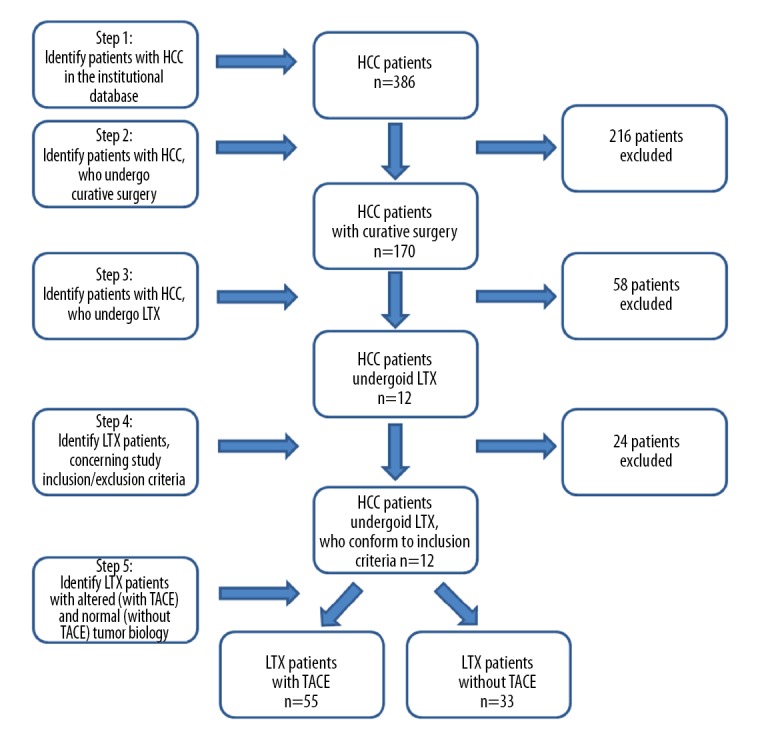
Flowchart depicting the patient selection process for our study.
Some of the HCC patients had a history of viral hepatitis (HBV or HCV). Antiviral therapy of the liver transplant recipients varied in respect to the type of viral hepatitis (HBV or HCV). Before LTX, patients with HBV received the nucleoside analogs lamivudine, entecavir, or tenofovir. Intraoperatively, 10 000 IE of hepatitis B immune globulins was administered. After LTX, the preoperatively initiated nucleoside analogs were maintained. Post-transplant monitoring of the anti-HBs titer and passive immunization, if <100 IE/L, were performed on a regular basis. Standard treatment of HCV transplant candidates before LTX consisted of PEGylated interferon (peg-IFN)-α and ribavirin. This regimen was maintained after the LTX. In some cases, peptidomimetic inhibitors (telaprevir) or protease inhibitors (boceprevir) were added.
Standard immunosuppressive regimens were administered after transplantation. All patients were initially given the calcineurin inhibitor tacrolimus. In 66/88 (75.0%) cases, the protocols also included the mTOR inhibitor everolimus. Due to adverse effects, in 16/88 (18.2%) cases tacrolimus was replaced by cyclosporine in the subsequent course. The standard protocols in all patients also included prednisolone in the initial phase after transplantation. This was tapered off and stopped 8 weeks after surgery. In some patients, mycophenolate mofetil was also given, but was stopped as soon as possible in the subsequent course.
Our study population comprised 2 cohorts: patients without (n=33) and patients with neoadjuvant TACE (n=55) prior to LTX. TACE in our institution was performed to sustain tumor control while bridging to transplantation. TACE procedures were performed using a standard protocol consisting of doxorubicin, mitomycin C, and lipiodol. In local anesthesia, angiography of the hepatic artery and subsequent selective visualization of tumor vessels was performed. In the next step, doxorubicin, mitomycin C, and lipiodol were selectively applied to the tumor vessels. In the control angiography and CT scan of the abdomen, depot of lipiodol with reduced and embolized vessels in the vicinity of the tumor were documented. TACE using drug-eluting beads (DEB) was performed in none of the cases.
Graft rejection was defined as rejection verified by histology after percutaneous liver biopsy using Banff criteria, or suspected rejection with improvement of liver function and enzymes after methylprednisolone i.v. therapy for at least 72 h. In all patients, hepatectomy and LTX were performed with curative intent. Paraffin-embedded, formalin-fixed tumor samples with a representative HCC specimen were retrieved from the Institute of Pathology. Experienced researchers (KD and GA) in histopathology carried out the histological and immunohistological analyses. An independent pathologist (KS) supervised the assessment process. The evaluation was carried out without knowledge of the outcome of the patients or the corresponding patient characteristics.
Immunohistology and tumor tissue densities analysis of angiopoietins and cellular immunologic infiltrates
The protocols for immunohistology, scoring of immune cells, and patient assignment to different groups for statistical assessment were carried out as previously described [14–16]. Briefly, we evaluated the tumor slice thoroughly for positive antibody staining, whereby the tumor infiltrating front (TIF) and the tumor central area (TCA) were examined separately. Positive staining for cellular infiltrates and angiopoietins seen in up to 5% of the tumor tissue was designated as negative/absent (0–5% positive cells, score 0), whereas >5% was designated as positive/present (>5% positive cells, score 1). Then, we grouped the patients by the presence or absence of the various immunologic entities and angiopoietins (Table 1).
Occurrence of histologic tumor necrosis
The appearance of tumor necrosis in HCC tissues was categorized into 1 for negative or 2 for positive, and then the liver transplants were assigned to a Necrosis+ or Necrosis− group, according to their ‘positive’ or ‘negative’ necrosis scores [17,18].
Statistical analysis
We used SPSS statistical software to conduct the univariate and survival analysis. A chi-squared test (χ2 test) was used to determine whether there was a significant difference between the observed frequencies in the categorical variables (clinicopathologic characteristics) in our study. Patient survival was analyzed with the log-rank test. Significant values from the univariate analysis and the ones that demonstrated an outlined trend towards significance were further processed in a Cox regression analysis model in a step-wise manner. Censoring in the survival analysis was performed if the event of interest (death of the patient) was not observed because of termination of the study observation period before the recruited subjects had shown the event of interest. These were 25 cases in the group of patients without TACE (25/33; 75.8%), and 42 cases (42/55; 76.4%) in the TACE group. Statistically significant values were assumed for p≤0.05.
Results
Table 1 provides a summary of the clinical background, underlying liver disease, and demographics of the patients included in the study. The underlying liver disease in 59/88 (67.0%) patients was alcoholic liver cirrhosis. NASH was diagnosed in 9/88 (10.2%) patients. Viral hepatitis was detected in 19/88 (21.6%) cases. Hemochromatosis was seen in 2/88 (2.2%) patients. Tumor recurrence with lethal complication proved to be the most common cause of death in the transplant patients. In patients receiving TACE, in 6/55 (10.9%) cases, local HCC recurrence in the graft was diagnosed. Graft HCC recurrence with concomitant metastatic spread was observed in 5/55 (9.0%) patients. Another 4/55 (7.2%) patients developed metastases to distant organs without evidence of local graft tumor recurrence. Altogether, 9/55 (16.4%) cases showed metastatic disease. In 13/55 (23.6%) cases, death occurred in the subsequent course. In 8/55 (14.5%) patients, the cause of death was HCC recurrence leading to lethal complications. In another 2/55 (3.6%) patients, graft failure due to recurrent viral hepatitis leading to complications of graft cirrhosis and liver failure were the main cause of death. Post-transplant gastric cancer with metastatic spread was the ultimate cause of death in 1/55 (1.8%) patients. A lethal Fournier’s gangrene was documented in 1/55 (1.8%) patients. In 1 case, the cause of death remained unknown. In the group of patients without TACE, local HCC recurrence with metastatic spread was observed in 4/33 (12.1%) patients. Another 5/33 (15.1%) patients had local recurrence without distant metastases. In 8/33 (24.2%) cases, patient death was documented in the subsequent course. Recurrent HCC with lethal complications was the cause of death in 5/33 (15.1%) patients, and fulminant stroke was observed in 1/33 (3.0%) patients. In 2 cases the cause of death remained unknown.
Host hepatic factors of angiogenesis are associated with graft rejection and M2-polarized macrophages
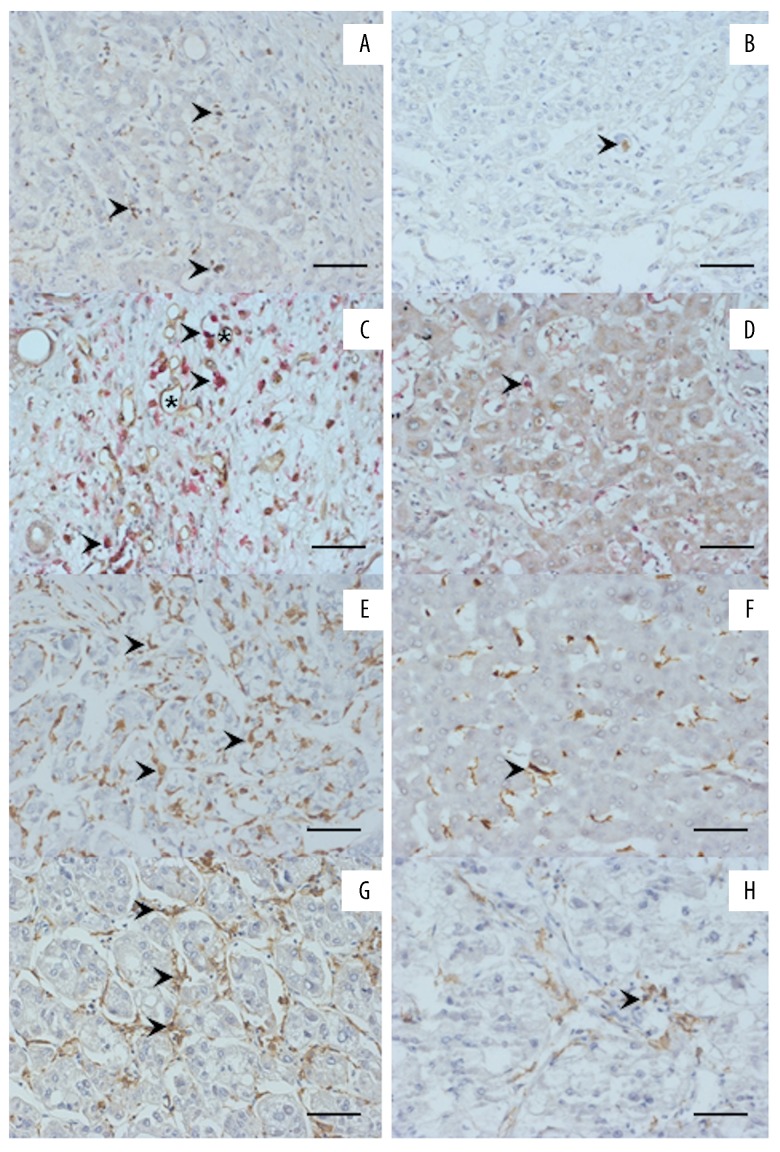
Clinicopathological characteristics of patients with and without neoadjuvant TACE are summarized in Table 1. As expected, patients undergoing TACE had smaller tumors (≤20 mm) and more advanced T stage prior to LTX, and had decreased incidence of distant metastasis and graft rejection after LTX. Figure 2 provides example photographs of angiopoietins and cellular immunologic infiltrates in the explanted host liver. Tables 1–5 give a synopsis of the statistical evaluation of all patients.
Figure 2.
Immunohistology for cellular infiltrates and angiopoietins in the host tumor central area (TCA) prior to liver transplantation for HCC (LTX) (left/right column: images of a typical patient/group with/without the respective positive staining are shown). The arrows point to positive staining; microvessels are indicated by asterisks; scale bar 50 μm. (A) High angiopoietin-1 frequency. (B) Low angiopoietin-1 frequency. (C) High frequency of TEMs. (D) Low frequency of TEMs. (E) High frequency of CD68+ TAMs. (F) Low frequency of CD68+ monocytes/macrophages. (G) High frequency of CD163+ monocytes/macrophages. (H) Low frequency of CD163+ monocytes/macrophages.
Table 2.
Group assignment of the patients undergoing liver transplantation for hepatocellular carcinoma in our study.
| Assessment of | Tumour area | TACE | Positive/presence | Negative/absence |
|---|---|---|---|---|
| Ang-2 | TIF | Without | ANG2+ n=25 | ANG2− n=8 |
| Ang-2 | TIF | With | ANG2+ n=38 | ANG2− n=17 |
| Ang-1 | TIF | Without | ANG1+ n=19 | ANG1− n=14 |
| Ang-1 | TIF | With | ANG1+ n=17 | ANG1− n=38 |
| TEMs | TCA | Without | TEM+ n=12 | TEM− n=21 |
| TEMs | TCA | With | TEM+ n=12 | TEM− n=43 |
| CD68+ TAMs | TCA | Without | CD68+ n=12 | CD68− n=21 |
| CD68+ TAMs | TCA | With | CD68+ n=16 | CD68− n=39 |
| M2-polaryzed TAMs | TIF | Without | CD163+ n=28 | CD163− n=5 |
| M2-polaryzaed TAMs | TIF | With | CD163+ n=6 | CD163− n=49 |
Ang-2 – angiopoetin-2; Ang-1 – angiopoetin-1; TAM – tumor associated macrophage; TCA – tumor central area; TEM – Tie2-expressing monocyte; TIF – tumor infiltrating front.
Table 3.
Correlation of host hepatic angiopoietin-1 expression at the tumour-infiltrating front (TIF) or central area (TCA) with clinicopathological characteristics of the patients undergoing liver transplantation for hepatocellular carcinoma.
| Variable | Without TACE | With TACE | ||||
|---|---|---|---|---|---|---|
| ANG2+/TIF | ANG2−/TIF | p | ANG2+/TIF | ANG2−/TIF | p | |
| No. of patients | 25 | 8 | 38 | 17 | ||
| Age | ||||||
| ≤60 | 12 (52.6%) | 2 (25.0%) | 0.252 | 17 (44.7%) | 6 (35.3%) | 0.512 |
| <60 | 13 (47.4%) | 6 (75.0%) | 21 (55.3%) | 11 (64.7%) | ||
| Gender | ||||||
| Male | 19 (76.0%) | 7 (87.5%) | 0.489 | 31 (81.6%) | 16 (94.1%) | 0.223 |
| Female | 6 (24.0%) | 1 (12.5%) | 7 (18.4%) | 1 (5.9%) | ||
| Graft tumour recurrence | ||||||
| Positive | 2 (10.5%) | 3 (21.4%) | 0.388 | 3 (17.6%) | 3 (13.2%) | 0.284 |
| Negative | 17 (89.5%) | 11 (78.6%) | 35 (82.4%) | 14 (82.4%) | ||
| Distant metastases | ||||||
| Positive | 3 (12.0%) | 1 (12.5%) | 0.970 | 6 (15.8%) | 3 (17.6%) | 0.863 |
| Negative | 22 (88.0%) | 7 (87.5%) | 32 (84.2%) | 14 (82.4%) | ||
| Graft rejection | ||||||
| With | 15 (60.0%) | 1 (12.5%) | 0.019 | 9 (23.7%) | 4 (23.5%) | 0.990 |
| Without | 10 (40.0%) | 7 (87.5%) | 29 (76.7%) | 13 (76.5%) | ||
| Tumour size, mm | ||||||
| ≤20 | 15 (60.0%) | 611 (22.4%) | 0.618 | 30 (78.9%) | 14 (82.4%) | 0.770 |
| >20 | 10 (40.0%) | 638 (77.6%) | 8 (21.1%) | 3 (17.6%) | ||
| Angioinvasion | ||||||
| Positive | 3 (12.0%) | 1 (12.5%) | 0.970 | 3 (7.9%) | 3 (17.6%) | 0.284 |
| Negative | 22 (88.0%) | 67 (87.5%) | 35 (92.1%) | 14 (82.4%) | ||
| Lymphangiosis carcinomatosa | ||||||
| Positive | 1 (4.0%) | 0 (0.0%) | 0.566 | 4 (10.5%) | 3 (17.6%) | 0.464 |
| Negative | 24 (96.0%) | 68 (100.0%) | 34 (89.5%) | 14 (82.4%) | ||
| Histologic differentiation | ||||||
| G1 well | 5 (20.0%) | 1 (12.5%) | 0.632 | 21 (55.3%) | 8 (47.1%) | 0.573 |
| G2 moderate/G3 poor | 20 (80.0%) | 7 (87.5%) | 17 (44.7%) | 9 (52.9%) | ||
| Pathologic T stage | ||||||
| T1 | 21 (84.0%) | 7 (87.5%) | 0.810 | 18 (47.4%) | 9 (52.9%) | 0.702 |
| T2/T3 | 4 (16.0%) | 1 (12.5%) | 20 (52.6%) | 8 (47.1%) | ||
| Variable | Without TACE | With TACE | ||||
|---|---|---|---|---|---|---|
| ANG1+/TIF | ANG1−/TIF | p | ANG1+/TIF | ANG1−/TIF | p | |
| No. of patients | 19 | 14 | 17 | 38 | ||
| Age | ||||||
| ≤60 | 10 (52.6%) | 4 (28.6%) | 0.167 | 8 (47.1%) | 15 (39.5%) | 0.598 |
| <60 | 9 (47.4%) | 10 (71.4%) | 9 (52.9%) | 623 (60.5%) | ||
| Gender | ||||||
| Male | 13 (68.4%) | 13 (92.9%) | 0.09 | 15 (88.2%) | 32 (84.2%) | 0.696 |
| Female | 6 (31.6%) | 1 (7.1%) | 2 (11.8%) | 6 (15.8%) | ||
| Graft tumour recurrence | ||||||
| Positive | 2 (10.5%) | 3 (21.4%) | 0.388 | 1 (5.9%) | 5 (13.2%) | 0.424 |
| Negative | 17 (89.5%) | 11 (78.6%) | 616 (94.1%) | 33 (86.8%) | ||
| Distant metastases | ||||||
| Positive | 2 (10.5%) | 2 (14.3%) | 0.744 | 2 (11.8%) | 7 (18.4%) | 0.537 |
| Negative | 17 (89.5%) | 12 (85.7%) | 15 (88.2%) | 31 (81.6%) | ||
| Graft rejection | ||||||
| With | 10 (52.6%) | 6 (42.9%) | 0.579 | 3 (17.6%) | 10 (26.3%) | 0.484 |
| Without | 9 (47.4%) | 8 (57.1%) | 14 (82.4%) | 28 (73.7%) | ||
| Tumour size, mm | ||||||
| ≤20 | 9 (47.4%) | 10 (71.6%) | 0.167 | 2 (11.8%) | 29 (76.3%) | 0.307 |
| >20 | 10 (52.6%) | 4 (28.6%) | 615 (88.2%) | 9 (23.7%) | ||
| Angioinvasion | ||||||
| Positive | 3 (15.8%) | 1 (7.1%) | 0.452 | 3 (17.6%) | 3 (7.9%) | 0.284 |
| Negative | 16 (84.2%) | 13 (92.9%) | 614 (82.4%) | 35 (92.1%) | ||
| Lymphangiosis carcinomatosa | ||||||
| Positive | 0 (0.0%) | 1 (7.1%) | 0.237 | 2 (11.8%) | 5 (13.2%) | 0.886 |
| Negative | 19 (100.0%) | 13 (92.9%) | 615 (88.2%) | 33 (86.8%) | ||
| Histologic differentiation | ||||||
| G1 well | 9 (47.4%) | 6 (42.9%) | 0.797 | 8 (47.1%) | 21 (55.3%) | 0.573 |
| G2 moderate/G3 poor | 10 (52.6%) | 8 (57.1%) | 9 (52.9%) | 17 (44.7%) | ||
| Pathologic T stage | ||||||
| T1 | 15 (78.9%) | 13 (92.9%) | 0.271 | 8 (47.1%) | 19 (50.0%) | 0.840 |
| T2/T3 | 4 (21.1%) | 1 (7.1%) | 9 (52.9%) | 19 (50.0%) | ||
| CD163+ TAMs in TCA | ||||||
| Positive | 9 (47.4%) | 10 (71.6%) | 0.167 | 5 (29.4%) | 24 (63.2%) | 0.021 |
| Negative | 10 (52.6%) | 4 (28.6%) | 12 (70.6%) | 14 (36.8%) | ||
Table 4.
Correlation of host hepatic monocytes/macrophages subtypes at the tumour-central area (TCA) or tumour infiltrating front (TIF) with clinicopathological characteristics of patients undergoing liver transplantation for hepatocellular carcinoma.
| Variable | Without TACE | With TACE | ||||
|---|---|---|---|---|---|---|
| TEM+/TCA | TEM−/TCA | p | TEM+/TCA | TEM+/TCA | p | |
| No. of patients | 12 | 21 | 12 | 43 | ||
| Age | ||||||
| ≤60 | 5 (41.7%) | 9 (42.9%) | 0.947 | 2 (16.7%) | 21 (48.8%) | 0.395 |
| <60 | 7 (58.3%) | 12 (57.1%) | 10 (83.3%) | 22 (51.2%) | ||
| Gender | ||||||
| Male | 8 (66.7%) | 18 (85.7%) | 0.198 | 9 (75.0%) | 38 (88.4%) | 0.245 |
| Female | 4 (33.3%) | 63 (14.3%) | 3 (25.0%) | 5 (11.6%) | ||
| Graft tumour recurrence | ||||||
| Positive | 2 (16.7%) | 3 (14.3%) | 0.854 | 1 (8.3%) | 5 (11.6%) | 0.746 |
| Negative | 10 (83.3%) | 18 (85.7%) | 11 (91.7%) | 38 (88.4%) | ||
| Distant metastases | ||||||
| Positive | 2 (16.7%) | 2 (9.5%) | 0.545 | 3 (25.0%) | 6 (14.0%) | 0.360 |
| Negative | 10 (83.3%) | 19 (90.5%) | 9 (75.0%) | 37 (86.0%) | ||
| Graft rejection | ||||||
| With | 4 (33.3%) | 12 (57.1%) | 0.188 | 2 (16.7%) | 11 (25.6%) | 0.520 |
| Without | 8 (66.7%) | 9 (42.9%) | 10 (83.3%) | 32 (74.4%) | ||
| Tumour size, mm | ||||||
| ≤20 | 4 (33.3%) | 15 (71.4%) | 0.033 | 8 (66.7%) | 36 (83.7%) | 0.192 |
| >20 | 8 (66.7%) | 6 (28.6%) | 4 (33.3%) | 7 (16.3%) | ||
| Angioinvasion | ||||||
| Positive | 2 (16.7%) | 2 (9.5%) | 0.545 | 2 (16.7%) | 4 (9.3%) | 0.469 |
| Negative | 10 (83.3%) | 619 (90.5%) | 10 (83.3%) | 39 (90.7%) | ||
| Lymphangiosis carcinomatosa | ||||||
| Positive | 0 (0.0%) | 1 (4.8%) | 0.443 | 3 (25.0%) | 4 (9.3%) | 0.149 |
| Negative | 12 (100.0%) | 20 (95.2%) | 9 (75.0%) | 39 (90.7%) | ||
| Histologic differentiation | ||||||
| G1 well | 5 (41.7%) | 10 (47.6%) | 0.741 | 6 (50.0%) | 23 (53.5%) | 0.831 |
| G2 moderate/G3 poor | 7 (58.3%) | 11 (52.4%) | 6 (50.0%) | 20 (46.5%) | ||
| Pathologic T stage | ||||||
| T1 | 10 (83.3%) | 18 (85.7%) | 0.854 | 6 (50.0%) | 21 (48.8%) | 0.943 |
| T2/T3 | 2 (16.7%) | 3 (14.3%) | 6 (50.0%) | 22 (51.2%) | ||
| Variable | Without TACE | With TACE | ||||
|---|---|---|---|---|---|---|
| CD68+/TCA | CD68−/TCA | p | CD68+/TCA | CD68−/TCA | p | |
| No. of patients | 12 | 21 | 16 | 39 | ||
| Gender | ||||||
| ≤60 | 5 (41.7%) | 9 (42.9%) | 0.947 | 3 (18.8%) | 20 (51.3%) | 0.026 |
| <60 | 7 (58.3%) | 12 (57.1%) | 13 (81.3%) | 19 (48.7%) | ||
| Gender | ||||||
| Male | 9 (75.0%) | 17 (81.0%) | 0.687 | 13 (81.3%) | 34 (87.2%) | 0.571 |
| Female | 3 (25.0%) | 4 (19.0%) | 3 (18.8%) | 5 (12.8%) | ||
| Graft tumour recurrence | ||||||
| Positive | 3 (25.0%) | 2 (9.5%) | 0.854 | 1 (6.3%) | 5 (12.8%) | 0.478 |
| Negative | 9 (75.0%) | 19 (90.5%) | 15 (93.8%) | 34 (87.2%) | ||
| Distant metastases | ||||||
| Positive | 2 (16.7%) | 2 (9.5%) | 0.545 | 1 (6.3%) | 8 (20.5%) | 0.194 |
| Negative | 10 (83.3%) | 19 (90.5%) | 15 (93.8%) | 31 (79.5%) | ||
| Graft rejection | ||||||
| With | 6 (50.0%) | 10 (47.6%) | 0.895 | 3 (18.8%) | 611 (25.6%) | 0.585 |
| Without | 6 (50.0%) | 11 (52.4%) | 13 (81.3%) | 32 (74.4%) | ||
| Tumour size, mm | ||||||
| ≤20 | 4 (33.3%) | 15 (71.4%) | 0.033 | 15 (93.8%) | 29 (74.4%) | 0.102 |
| >20 | 8 (66.7%) | 6 (28.6%) | 1 (6.3%) | 10 (25.6%) | ||
| Angioinvasion | ||||||
| Positive | 1 (8.3%) | 3 (14.3%) | 0.545 | 2 (12.5%) | 4 (10.3%) | 0.808 |
| Negative | 11 (91.7%) | 18 (85.7%) | 14 (87.5%) | 35 (89.7%) | ||
| Lymphangiosis carcinomatosa | ||||||
| Positive | 0 (0.0%) | 1 (4.8%) | 0.443 | 0 (0.0%) | 7 (17.9%) | 0.070 |
| Negative | 12 (100.0%) | 20 (95.2%) | 16 (100.0%) | 632 (82.1%) | ||
| Histologic differentiation | ||||||
| G1 well | 8 (66.7%) | 7 (33.3%) | 0.064 | 9 (56.3%) | 20 (53.5%) | 0.737 |
| 6G2 moderate/G3 poor | 4 (33.3%) | 14 (66.7%) | 7 (43.8%) | 619 (48.7%) | ||
| Pathologic T stage | ||||||
| T1 | 18 (85.7%) | 10 (83.3%) | 0.854 | 12 (75.0%) | 15 (38.5%) | 0.014 |
| T2/T3 | 3 (14.3%) | 2 (16.7%) | 4 (25.0%) | 24 (61.5%) | ||
| TEMs in TCA | ||||||
| Positive | 13 (61.9%) | 8 (66.7%) | 0.544 | 34 (87.2%) | 9 (56.3%) | 0.012 |
| Negative | 8 (38.1%) | 4 (33.3%) | 5 (12.8%) | 7 (43.8%) | ||
| No. of patients | 5 | 28 | 45 | 10 | ||
| Angioinvasion | ||||||
| Positive | 2 (40.0%) | 2 (7.1%) | 0.038 | 4 (8.9%) | 2 (20.0%) | 0.308 |
| Negative | 3 (60.0%) | 26 (92.9%) | 41 (91.1%) | 68 (80.0%) | ||
| Histologic differentiation | ||||||
| G1 well | 2 (40.0%) | 13 (46.4%) | 0.790 | 27 (60.0%) | 2 (20.0%) | 0.022 |
| G2 moderate/G3 poor | 3 (60.0%) | 15 (53.6%) | 18 (40.0%) | 8 (80.0%) | ||
| Variable | Without TACE | With TACE | ||||
|---|---|---|---|---|---|---|
| CD163+/TIF | CD163−/TCA | p | CD163+/TIF | CD163−/TCA | p | |
| No. of patients | 28 | 5 | 6 | 49 | ||
| Distant metastases | ||||||
| Positive | 2 (7.1%) | 2 (40.0%) | 0.038 | 0 (00.0%) | 9 (18.4%) | 0.251 |
| Negative | 26 (92.9%) | 3 (60.0%) | 6 (100.0%) | 40 (81.6%) | ||
Table 5.
Correlation of host hepatic tumour necrosis with clinicopathological characteristics of the patients undergoing liver transplantation for hepatocellular carcinoma.
| Variable | Without TACE | With TACE | ||||
|---|---|---|---|---|---|---|
| Necrosis+ | Necrosis− | p | Necrosis+ | Necrosis− | p | |
| No. of patients | 9 | 24 | 46 | 9 | ||
| Age | ||||||
| ≤60 | 5 (55.6%) | 9 (37.5%) | 0.350 | 21 (45.7%) | 2 (22.2%) | 0.193 |
| <60 | 4 (44.4%) | 15 (62.5%) | 25 (54.3%) | 7 (77.8%) | ||
| Gender | ||||||
| Male | 7 (77.8%) | 19 (79.2%) | 0.931 | 39 (84.8%) | 8 (88.9%) | 0.749 |
| Female | 2 (22.2%) | 5 (20.8%) | 7 (15.2%) | 1 (11.1%) | ||
| Graft tumour recurrence | ||||||
| Positive | 2 (22.2%) | 3 (12.5%) | 0.488 | 3 (6.5%) | 3 (33.3%) | 0.018 |
| Negative | 7 (77.8%) | 21 (87.5%) | 43 (93.5%) | 6 (66.7%) | ||
| Distant metastases | ||||||
| Positive | 2 (22.2%) | 2 (8.3%) | 0.276 | 6 (13.0%) | 3 (33.3%) | 0.132 |
| 6Negative | 7 (77.8%) | 22 (91.7%) | 40 (87.0%) | 6 (66.7%) | ||
| Graft rejection | ||||||
| With | 4 (44.4%) | 12 (50.0%) | 0.776 | 12 (26.1%) | 1 (11.1%) | 0.333 |
| Without | 5 (55.6%) | 12 (50.0%) | 34 (73.9%) | 8 (88.9%) | ||
| Tumour size, mm | ||||||
| ≤20 | 3 (33.3%) | 16 (66.7%) | 0.084 | 35 (76.1%) | 9 (100.0%) | 0.101 |
| >20 | 6 (66.7%) | 8 (33.3%) | 11 (23.9%) | 0 (00.0%) | ||
| Angioinvasion | ||||||
| Positive | 2 (22.2%) | 2 (8.3%) | 0.276 | 6 (13.0%) | 0 (00.0%) | 0.251 |
| Negative | 7 (77.8%) | 22 (91.7%) | 40 (87.0%) | 9 (100.0%) | ||
| Lymphangiosis carcinomatosa | ||||||
| Positive | 0 (0.0%) | 1 (4.2%) | 0.534 | 6 (13.0%) | 1 (11.1%) | 0.874 |
| Negative | 9 (100.0%) | 23 (95.8%) | 40 (87.0%) | 8 (88.9%) | ||
| Histologic differentiation | ||||||
| G1 well | 3 (33.3%) | 12 (50.0%) | 0.392 | 25 (54.3%) | 4 (44.4%) | 0.586 |
| G2 moderate/G3 poor | 6 (66.7%) | 12 (50.0%) | 21 (45.7%) | 5 (55.6%) | ||
| Pathologic T stage | ||||||
| T1 | 6 (66.7%) | 22 (91.7%) | 0.074 | 26 (56.5%) | 1 (11.1%) | 0.013 |
| T2/T3 | 3 (33.3%) | 2 (8.3%) | 20 (43.5%) | 8 (88.9%) | ||
| CD163+ TAMs in TCA | ||||||
| Positive | 4 (44.4%) | 15 (62.5%) | 0.350 | 21 (45.7%) | 8 (88.9%) | 0.018 |
| Negative | 5 (55.6%) | 9 (37.5%) | 25 (54.3%) | 1 (11.1%) | ||
| Angiopoietin-1/TIF | ||||||
| Positive | 8 (88.9%) | 11 (45.8%) | 0.026 | 31 (67.4%) | 7 (77.8%) | 0.537 |
| Negative | 1 (11.1%) | 13 (54.2%) | 15 (32.6%) | 2 (22.2%) | ||
The presence of angiopoietin-2 at the TIF in the explanted liver was associated with graft rejection after LTX (p=0.019; Figure 2A, 2B; Table 3). In the Ang2− group, only 1/8 (12.5%) patients had organ rejection, but in the Ang2+ group, 15/25 (25.0%) patients suffered organ rejection. In HCC patients who received TACE prior to LTX, the angiopoietins in the TCA or at TIF were associated with the presence of CD163+ TAMs (Table 3). In the Ang1+ group, 12/17 (70.6%) patients showed CD163+ TAMs at the TIF, but in the Ang1− group only 14/38 (36.8%) patients showed CD163+ TAMs at the TIF (p=0.021).
Monocytes/macrophages are associated with tumor size, grading, angioinvasion, and metastatic disease
In patients without TACE, TEMs and CD68+ TAMs were associated with a larger tumor size (Figure 2C–2F, Table 4). In the TEM− group, 15/21 (71.4%) patients had a smaller tumor size, whereas in the TEM+ group only 4/12 (33.3%) patients had this feature (p=0.033). In addition, the presence of CD68+ TAMs in TCA revealed an identical phenomenon (p=0.033; Table 4). In the TIF, CD68+ TAMs were associated with more frequent angioinvasion (p=0.038). In the CD68− group, angioinvasion was detected in 2/28 (7.1%) patients. This was also the case in 2/5 (40%) patients in the CD68+ group. Moreover, CD163+ TAMs in TIF were associated with a more frequent metastasis after LTX (p=0.038). In the CD163− group, 2/28 (7.1%) patients had distant metastases, but in the CD163+ group 3/5 (60.0%) patients had distant metastases, when considering the TIF (p=0.038; Table 4).
In patients who received TACE prior to LTX, monocytes/macrophages were associated with a more advanced tumor grading (Table 4). In the CD68+ group, 8/10 (80.0%) patients had G2/G3 tumors. In the CD68− group, only 18/45 (40.0%) patients revealed this characteristic (p=0.022). Interestingly, the presence of CD68+ TAMs and TEMs in these pre-treated tumors were strongly associated: in the CD68+ group 34/39 (87.2%) patients showed TEMs in the TCA, but in the CD68− group 9/16 (56.3%) patients showed TEMs in the TCA (p=0.012).
Tumor necrosis is associated with angiopoietin expression, M2-polarized TAMs, and reduced incidence of tumor recurrence
24/33 (72.7%) patients without TACE showed no tumor necrosis in the HCC group (Table 5). High expression of angiopoietin-1 in tumor cells in the TIF was associated with tumor necrosis: in the Ang1+ group 8/9 (88.9%) of the HCC showed tumor necrosis, but in the Ang1− group 11/24 (45.8%) of the HCC showed tumor necrosis (p=0.026).
In 46/55 (83.6%) of the patients with TACE prior to LTX, tumor necrosis was detected and was associated with enhanced infiltration of CD163+ TAMs (p=0.018; Table 5). In the Necrosis+ group 25/46 (54.3%) patients showed TEMs in the TCA, but in the Necrosis− group only 1/9 (11.1%) patients showed TEMs in the TCA. Furthermore, tumor necrosis was associated with a lower incidence of recurrent graft HCC after TACE and LTX (p=0.018). In the Necrosis+ group only in 3/46 (6.5%) patients had tumor necrosis, but in the Necrosis− group the tumor recurred in 3/9 (33.3%) patients.
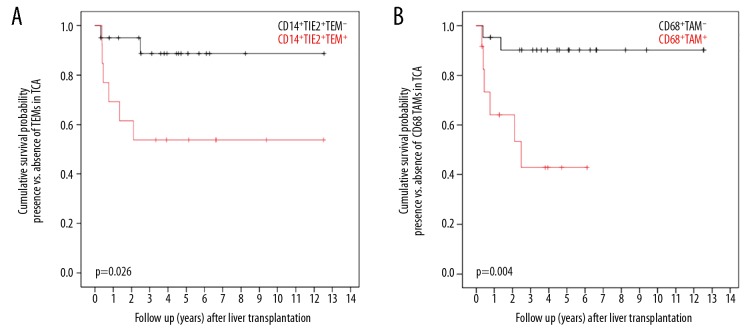
Presence of monocytes/macrophages in the host liver is associated with survival after transplantation
Recipient hepatic TEMs and CD68+ TAMs were associated with patient overall survival after LTX without TACE (Figure 3, Tables 1–5). In the TCA, TEMs were associated with lower patient survival (p=0.026). At 1, 3, and 5 years after LTX, 70.8%, 56.8%, and 56.8%, respectively, of patients in the TEM+ group were alive, in comparison to 93.3%, 88.2%, and 88.2%, respectively, of patients in the TAM− group (Figure 3A). Furthermore, CD68+ TAMs in TCA were associated with worse patient survival (p=0.026; figure 3B): At 1, 3, and 5 years after surgery, the overall survival was 63.8%, 42.6%, and 42.6%, respectively, in patients with, but it was 94.6%, 90.1%, and 90.1%, respectively, in patients without CD68+ TAMs at the TCA.
Figure 3.
(A) Overall survival after LTX in relation to occurrence of host hepatic TEMs. (B) Overall survival after LTX in relation to occurrence of host hepatic CD68+ monocytes/macrophages.
Discussion
Our study assessed the relationship of presence of angiopoietin-positive tumor cells, infiltrating monocytes/macrophages, and tumor necrosis in HCC samples from the recipient liver prior to LTX with tumor recurrence, survival, and graft rejection. Nearly two-third of the patients were pre-treated with TACE. We hypothesized that, in the tumor microenvironment of HCC, monocytes/macrophages, angiopoietins, and tumor necrosis are coherently interconnected and can influence the outcome after oncologic LTX. The main findings were: (1) Monocytes/macrophages in the recipient native liver prior to LTX influence survival after transplantation; (2) when receiving TACE, development of tumor necrosis was associated with lower incidence of recurrent HCC following LTX and with enhanced expression of monocytes/macrophages and angiopoietin-2; (3) pre-transplant expression of angiopoietin-2 in tumor cells was associated to more frequent graft rejection after LTX; (4) monocytes/macrophages were associated with angioinvasion, tumor size, and metastatic disease.
HCC is the hallmark of inflammation-associated cancer. Tumor-related escape mechanisms such as inadequate tumor clearance and suppression of anti-tumor immunity are among the most challenging paradigms in hepatology [19]. In chronic hepatic inflammation, infiltrating monocytes/macrophages in the tumor microenvironment have long been recognized as essential drivers of tumor progression [20]. Cancer is related with major immunosuppression of the host, which is significantly aggravated after LTX. There is little data on the importance of host immunologic competence in the tumor microenvironment in clinical outcome following solid organ transplantation. Here, we demonstrated that host hepatic monocytes/macrophages, angiogenesis-related factors, and occurrence of post-interventional tumor necrosis are coherently related and exert a significant effect on patient outcome after LTX.
Our study demonstrated that high abundance of angiopoietin in the explanted recipient’s liver was associated with graft rejection after oncologic LTX. Scientific data on the importance of angiopoietins in solid organ transplantation are scarce. However, recent data suggest there are functional links between angiopoietins and enhanced immunologic infiltration [21]. In the present study of oncologic LTX, angiopoietin expression was found to be associated with increased infiltration of monocytes/macrophages. Thus, elevated expression of recipient’s factors of angiogenesis can facilitate enhanced infiltration of the graft with immune-competent and antigen-presenting cells after transplantation. This phenomenon can trigger immunologic mechanisms that ultimately lead to increased incidence of organ rejection. These findings suggest that angiopoietin signaling influences infiltration of monocytes/macrophages in HCC, leading to worse disease outcomes; however, additional research is warranted to elucidate the mechanistic links.
In the context of hepatocarcinogenesis, HCC-associated monocyte/macrophage subsets are novel targets of immunologic checkpoint inhibition, and in vivo targeting of myeloid cell populations that control crucial hepatic inflammatory responses might be new approaches for efficient drug delivery [22]. In addition, manipulating the role of CD68-positive Kupffer cells in regulating the initial hepatic injury, aggravating the immunologic cascades by infiltrating monocytes, and their consequent re-/programming into tumor-promoting M2-polarized phenotypes, are promising targets for novel therapeutic interventions. In our study, we demonstrated that TEMs and CD68+ macrophages are significantly associated with multiple tumor characteristics and worse survival after LTX. Of note, the experimental depletion of Kupffer cells in vivo was demonstrated to attenuate hepatocarcinogenesis via cytokeratin 19/Oval-dependent pathways, which was related to improved response to treatment with sorafenib in this subgroup of patients [23]. TEMs were shown to indicate the effect of sorafenib therapy and to serve as a complementary biomarker for α-fetoprotein in diagnosing α-fetoprotein-negative advanced HCC [24,25]. Furthermore, TEMs were demonstrated to directly respond to angiopoietin activity [26]. In experimental cancer models, selective depletion of TEMs and corresponding angiopoietin-related pathways significantly impaired tumor growth and angiogenesis [27].
Recent data indicate that the occurrence of histologic tumor necrosis is functionally mediated by infiltrating monocytes/macrophages [28–30]. Moreover, the utilization of bridging therapy such as TACE prior to LTX is associated with the extent of tumor necrosis of the recipient liver and supports the essential role of chemotherapy-induced cytotoxicity in TACE effectiveness [31]. We found that formation of host histologic tumor necrosis after TACE was associated with enhanced presence of monocytes/macrophages and reduced incidence of recurrent HCC after LTX. In this scenario, infiltrating TAMs and tumor necrosis represent important integrational components of a common phenomenon in tumor-related hepatic inflammation. In the oncologic setting, this construct comprising monocytes/macrophages and tumor necrosis may help to identify a patient subgroup with favorable characteristics and to predict their outcome after TACE and LTX.
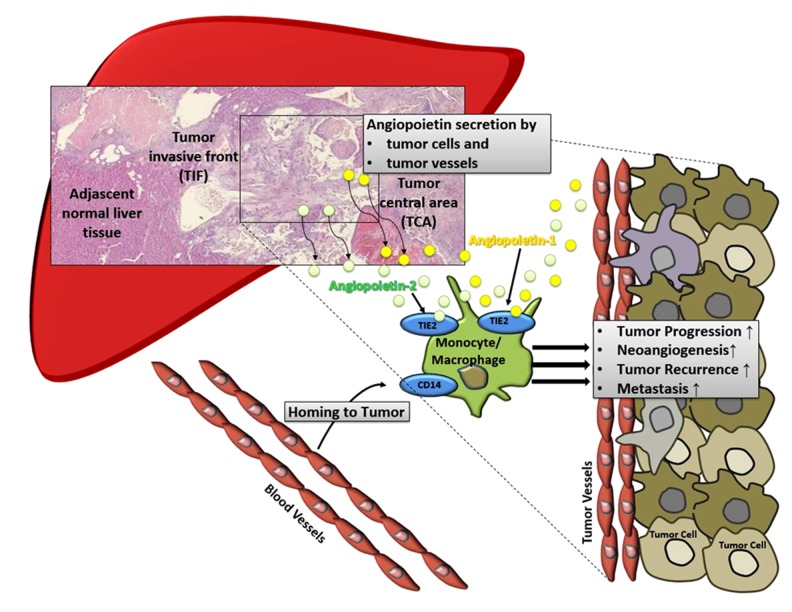
Previous results published by our group revealed an important role of various components of the tumor microenvironment, such as infiltrating subsets of monocytes/macrophages, angiogenic biomarkers, and formation of necrosis in regard to tumor progression. Based on these findings, in the current work we hypothesized that in HCC prior transplantation, monocytes/macrophages and related factors of angiogenesis exert a significant effect on clinical outcome after LTX. We conceived a possible functional link, in which angiopoietins released by malignant cells and endothelium on tumor blood vessels mediate homing of monocytes/macrophages to the tumor microenvironment of HCC via the TIE2 receptors. Invading monocytes/macrophages then orchestrate the formation of histologic tumor necrosis, promote tumor escape mechanisms, and ultimately cause cancer progression (Figure 4). Interestingly, in our study, these immunologic characteristics in the recipient native liver prior transplantation (i.e., the surgical removal of the HCC and related tumor microenvironment) were associated with the outcome after transplantation. However, this study has certain limitations. The relatively small study cohort and corresponding number of cases in the various subgroups are a major drawback. We demonstrated strong statistical associations, but the nature of our work remains descriptive. Thus, experimental mechanistic and functional downstream research will help clarify the causal pathways presumed in the present work.
Figure 4.
We hypothesized that angiopoietin-related molecular links mediate homing of TEMs to the microenvironment of HCC utilizing the TIE2-axis. These tumor-infiltrating monocytes/macrophages are then responsible for the establishment of necrosis, further tumor escape mechanisms, and cancer growth.
Conclusions
In the context of altered recipient immunologic competence after solid organ transplantation, there is scant data on the role of the angiopoietin axis, tumor necrosis, and monocytes/macrophages. Our study demonstrated a coherent pre-transplant construct composed of recipient hepatic angiogenic factors, monocytes/macrophages, and tumor necrosis associated with numerous clinicopathologic variables and disease outcome after LTX for HCC. However, possible limitations should be taken into consideration, as well. The small number of cases with liver transplantation on grounds of underlying HCC represent the main obstacle. The descriptive nature of our findings may also be of potential limitation. Thus, further research is warranted to elucidate the possible functional links, which could help identify novel targets of immunologic checkpoint inhibition in liver cancer.
Abbreviations
- Ang
angiopoietin
- HCC
hepatocellular carcinoma
- TAM
tumor-associated macrophage
- TACE
transarterial chemoembolization
- TCA
tumor central area
- TEM
TIE2-expressing monocyte
- TIF
tumor infiltrating front
Footnotes
Source of support: This work was made possible by funding from the Berlin Institute of Health (BIH) to GA. Georgi Atanasov is a participant of the BIH Charité Clinician Scientist Program funded by the Charité–Universitätsmedizin Berlin and the BIH
Conflict of interest
None.
References
- 1.Ghouri YA, Mian I, Rowe JH. Review of hepatocellular carcinoma: Epidemiology, etiology, and carcinogenesis. J Carcinog. 2017;16:1. doi: 10.4103/jcar.JCar_9_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kulik L, Heimbach JK, Zaiem F, et al. Therapies for patients with hepatocellular carcinoma awaiting liver transplantation: A systematic review and meta-analysis. Hepatology. 2018;67(1):381–400. doi: 10.1002/hep.29485. [DOI] [PubMed] [Google Scholar]
- 3.Bishayee A. The role of inflammation and liver cancer. Adv Exp Med Biol. 2014;816:401–35. doi: 10.1007/978-3-0348-0837-8_16. [DOI] [PubMed] [Google Scholar]
- 4.Pathria P, Louis TL, Varner JA. Targeting tumor-associated macrophages in cancer. Trends Immunol. 2019;40(4):310–27. doi: 10.1016/j.it.2019.02.003. [DOI] [PubMed] [Google Scholar]
- 5.Yeung OW, Lo CM, Ling CC, et al. Alternatively activated (M2) macrophages promote tumour growth and invasiveness in hepatocellular carcinoma. J Hepatol. 2015;62(3):607–16. doi: 10.1016/j.jhep.2014.10.029. [DOI] [PubMed] [Google Scholar]
- 6.Mantovani A, Marchesi F, Malesci A, et al. Tumour-associated macrophages as treatment targets in oncology. Nat Rev Clin Oncol. 2017;14(7):399–416. doi: 10.1038/nrclinonc.2016.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bredholt G, Mannelqvist M, Stefansson IM, et al. Tumor necrosis is an important hallmark of aggressive endometrial cancer and associates with hypoxia, angiogenesis and inflammation responses. Oncotarget. 2015;6(37):39676–91. doi: 10.18632/oncotarget.5344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eilber FC, Rosen G, Eckardt J, et al. Treatment-induced pathologic necrosis: A predictor of local recurrence and survival in patients receiving neoadjuvant therapy for high-grade extremity soft tissue sarcomas. J Clin Oncol. 2001;19(13):3203–9. doi: 10.1200/JCO.2001.19.13.3203. [DOI] [PubMed] [Google Scholar]
- 9.Villa E, Critelli R, Lei B, et al. Neoangiogenesis-related genes are hallmarks of fast-growing hepatocellular carcinomas and worst survival. Results from a prospective study. Gut. 2016;65(5):861–69. doi: 10.1136/gutjnl-2014-308483. [DOI] [PubMed] [Google Scholar]
- 10.Gillen J, Richardson D, Moore K. Angiopoietin-1 and Angiopoietin-2 inhibitors: Clinical development. Curr Oncol Rep. 2019;21(3):22. doi: 10.1007/s11912-019-0771-9. [DOI] [PubMed] [Google Scholar]
- 11.Yang P, Chen N, Yang D, et al. Cervical cancer cell-derived angiopoietins promote tumor progression. Tumour Biol. 2017;39(7) doi: 10.1177/1010428317711658. 1010428317711658. [DOI] [PubMed] [Google Scholar]
- 12.Campanelli R, Fois G, Catarsi P, et al. Tie2 expressing monocytes in the spleen of patients with primary myelofibrosis. PLoS One. 2016;11(6):e0156990. doi: 10.1371/journal.pone.0156990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Turrini R, Pabois A, Xenarios I, et al. TIE-2 expressing monocytes in human cancers. Oncoimmunology. 2017;6(4):e1303585. doi: 10.1080/2162402X.2017.1303585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Atanasov G, Dino K, Schierle K, et al. Immunologic cellular characteristics of the tumour microenvironment of hepatocellular carcinoma drive patient outcomes. World J Surg Oncol. 2019;17(1):97. doi: 10.1186/s12957-019-1635-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Atanasov G, Pötner C, Aust G, et al. TIE2-expressing monocytes and M2-polarized macrophages impact survival and correlate with angiogenesis in adenocarcinoma of the pancreas. Oncotarget. 2018;9(51):29715–26. doi: 10.18632/oncotarget.25690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Atanasov G, Dietel C, Feldbrügge L, et al. Angiogenic miRNAs, the angiopoietin axis and related TIE2-expressing monocytes affect outcomes in cholangiocarcinoma. Oncotarget. 2018;9(52):29921–33. doi: 10.18632/oncotarget.25699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Atanasov G, Schierle K, Hau HM, et al. Prognostic significance of tumor necrosis in hilar cholangiocarcinoma. Ann Surg Oncol. 2017;24(2):518–25. doi: 10.1245/s10434-016-5472-0. [DOI] [PubMed] [Google Scholar]
- 18.Atanasov G, Dietel C, Feldbrügge L, et al. Tumor necrosis and infiltrating macrophages predict survival after curative resection for cholangiocarcinoma. OncoImmunology. 2017;6(8):e1331806. doi: 10.1080/2162402X.2017.1331806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tacke F. Targeting hepatic macrophages to treat liver diseases. J Hepatol. 2017;66(6):1300–12. doi: 10.1016/j.jhep.2017.02.026. [DOI] [PubMed] [Google Scholar]
- 20.Del Campo JA, Gallego P, Grande L. Role of inflammatory response in liver diseases: Therapeutic strategies. World J Hepatol. 2018;10(1):1–7. doi: 10.4254/wjh.v10.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carbone C, Piro G, Merz V, et al. Angiopoietin-like proteins in angiogenesis, inflammation and cancer. Int J Mol Sci. 2018;19(2) doi: 10.3390/ijms19020431. pii:E431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ergen C, Heymann F, Al Rawashdeh W, et al. Targeting distinct myeloid cell populations in vivo using polymers, liposomes and microbubbles. Biomaterials. 2017;114:106–20. doi: 10.1016/j.biomaterials.2016.11.009. [DOI] [PubMed] [Google Scholar]
- 23.Li XF, Chen C, Xiang DM, et al. Chronic inflammation-elicited liver progenitor cell conversion to liver cancer stem cell with clinical significance. Hepatology. 2017;66(6):1934–51. doi: 10.1002/hep.29372. [DOI] [PubMed] [Google Scholar]
- 24.Mao L, Wang Y, Wang D, et al. TEMs but not DKK1 could serve as complementary biomarkers for AFP in diagnosing AFP-negative hepatocellular carcinoma. PLoS One. 2017;12(9):e0183880. doi: 10.1371/journal.pone.0183880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shoji H, Yoshio S, Mano Y, et al. Pro-angiogenic TIE-2-expressing monocytes/TEMs as a biomarker of the effect of sorafenib in patients with advanced hepatocellular carcinoma. Int J Cancer. 2017;141(5):1011–17. doi: 10.1002/ijc.30804. [DOI] [PubMed] [Google Scholar]
- 26.Murdoch C, Tazzyman S, Webster S, Lewis CE. Expression of Tie-2 by human monocytes and their responses to angiopoietin-2. J Immunol. 2007;178(11):7405–11. doi: 10.4049/jimmunol.178.11.7405. [DOI] [PubMed] [Google Scholar]
- 27.De Palma M, Murdoch C, Venneri MA, et al. Tie2-expressing monocytes: Regulation of tumor angiogenesis and therapeutic implications. Trends Immunol. 2007;28(12):519–24. doi: 10.1016/j.it.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 28.Ehling J, Bartneck M, Wei X, et al. CCL2-dependent infiltrating macrophages promote angiogenesis in progressive liver fibrosis. Gut. 2014;63:1960–71. doi: 10.1136/gutjnl-2013-306294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karlmark KR, Weiskirchen R, Zimmermann HW, et al. Hepatic recruitment of the inflammatory Gr1+ monocyte subset upon liver injury promotes hepatic fibrosis. Hepatology. 2009;50:261–74. doi: 10.1002/hep.22950. [DOI] [PubMed] [Google Scholar]
- 30.Seki E, Schwabe RF. Hepatic inflammation and fibrosis: Functional links and key pathways. Hepatology. 2015;61:1066–79. doi: 10.1002/hep.27332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gaba RC, Emmadi R, Parvinian A, Casadaban LC. Correlation of doxorubicin delivery and tumor necrosis after drug-eluting bead transarterial chemoembolization of rabbit VX2 liver tumors. Radiology. 2016;280(3):752–61. doi: 10.1148/radiol.2016152099. [DOI] [PubMed] [Google Scholar]