Abstract
Introduction
First-degree relatives of patients with familial aneurysmal subarachnoid hemorrhage have an increased risk of unruptured intracranial aneurysms and aneurysmal subarachnoid hemorrhage. We assessed whether the type of kinship of first-degree relatives of aneurysmal subarachnoid hemorrhage patients influences this risk.
Patients and methods
We used all available data from the prospectively collected database of families consulting our outpatient clinic between 1994-2016. We constructed pedigrees for all families with ≥2 first-degree relatives with aneurysmal subarachnoid hemorrhage or unruptured intracranial aneurysms. The proband was defined as the first family member with aneurysmal subarachnoid hemorrhage who sought medical attention. We compared both the proportion of aneurysmal subarachnoid hemorrhage and unruptured intracranial aneurysms in proband's first-degree relatives by calculating relative risks (RR) with children as the reference.
Results
We studied 154 families with 1,105 first-degree relatives of whom 146 had aneurysmalsubarachnoid hemorrhage. Unruptured intracranial aneurysms were identified in 63 (19%) of the 326 screened relatives. Siblings had a higher risk of aneurysmal subarachnoid hemorrhage (RR:1.62, 95% CI:1.12–2.38) and parents a lower risk (RR:0.44, 95% CI:0.24–0.81) than children. Siblings also had a higher risk of unruptured intracranial aneurysms (RR:2.28, 95% CI:1.23–4.07, age-adjusted RR:2.04, 95% CI:1.07–3.92) than children.
Conclusion: Siblings of patients with aneurysmal subarachnoid hemorrhage have a significanthigher risk of both unruptured intracranial aneurysms and aneurysmal subarachnoid hemorrhage and parents have a lower risk of aneurysmal subarachnoid hemorrhage than children.
Discussion: Type of kinship is a relevant factor to consider in risk prediction and screening advice in families with familial aneurysmal subarachnoid hemorrhage.
Keywords: Intracranial aneurysm, subarachnoid haemorrhage, familial
Introduction
Intracranial aneurysms are present in approximately 3% of the adult population.1 Rupture of an intracranial aneurysm results in aneurysmal subarachnoid haemorrhage (aSAH), which is a subtype of stroke that carries a high morbidity and fatality.2 A positive family history for aSAH is an important risk factor for aSAH. First-degree relatives of patients with aSAH have an increased risk of unruptured intracranial aneurysms (UIA) and aSAH.3,4 In 25% of persons with two or more affected first-degree relatives with aSAH, UIA are detected during life.5 Currently, a more tailored screening is not yet possible within relatives of patients with familial aSAH, as we are not able to further specify the risk of developing UIA. Consequently, we apply the same screenings programme to all first-degree relatives.
The type of kinship may influence the risk of UIA and of aSAH, with siblings having the highest risk, but studies so far have conflicting results which might be caused by the small number of families with familial aSAH in these studies.3,6–12 Furthermore, none of the studies assessed the risk of both UIA and aSAH together.
The aim of this study was to assess in a large study population whether the type of kinship (parents, siblings, or children) of the first-degree relatives of aSAH patients influences the risk for UIA and aSAH.
Patients and methods
Study population
All individuals who are screened at the University Medical Center (UMC) Utrecht, The Netherlands, for intracranial aneurysms because of familial aSAH are recorded in a prospectively collected database. A positive family history was defined as two or more first-degree relatives (parents, siblings, or children) with aSAH or UIA. All patients with aSAH who were admitted or individuals with an UIA who visited the outpatient clinic at the UMC Utrecht were routinely asked for details about their family history. If aSAH occurred in their relatives, we suggested that they extend an invitation to their relatives to visit the outpatient clinic to be informed about screening for UIA. Individuals were also referred for screening by their general practitioner or neurologist. We retrieved all available information from the period from April 1994 to December 2016.
Data collection
Pedigrees were constructed based on the familial history of the probands or relatives who presented for screening in the UMC Utrecht. For the purpose of our study, the proband was defined as the relative with aSAH who was first brought under medical attention. aSAH must have been identified in a hospital. We obtained information about age, sex, and familial and personal history of UIA or aSAH of all relatives from the database. All UIA in first-degree relatives were identified by CT, MRI, or conventional angiogram in the UMC Utrecht. Screening was usually performed from the age of 18 years until the age of approximately 70 years, with the precise cutoff depending on their state of health. In case of a negative screen, people were advised to contact us after 5 years to repeat screening. Individuals were not actively invited for repeated screening. Performed screening reflects clinical practice and was not according to a study protocol. Consequently, screening intervals shorter and longer than the advised 5 years could occur. Only relatives screened for UIA in the UMC Utrecht were included. We excluded patients with autosomal-dominant polycystic kidney disease. This study was approved by the institutional Research Ethics Board of the University Medical Center Utrecht.
Statistical analysis
To assess the association between type of kinship of a probands’ first-degree relative and risk of aSAH and UIA, we calculated proportions and relative risks (RRs) with corresponding 95% confidence intervals (CIs) with Poisson regression with children as the reference. For the analysis on the risk of UIA, we included only proband’s relatives screened for UIA. This analysis was repeated with adjustment for age. Adjustment for age was not possible in our analysis to assess the association between type of kinship of a probands’ first-degree relative and risk of aSAH, as data on age were missing in >70% of all first-degree relatives.
Results
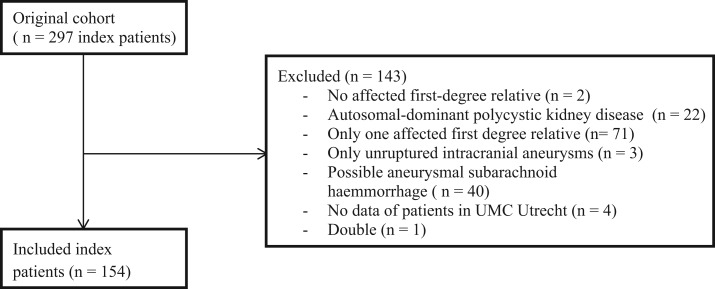
We studied 154 families (Figure 1) with a total of 1105 proband’s first-degree relatives (Table 1). The mean number of relatives per family was 7 (range: 3–23). Of those 1105 relatives, 146 had an aSAH and 326 relatives were screened for UIA, with UIA identified in 63 (19%) of them. The mean duration of follow-up of screened relatives was 87 ± 80 months in siblings and 69 ± 76 months in children. The mean age at time of UIA diagnosis was 47 years in children and 52 years in siblings.
Figure 1.
Flow chart of included patients.
Table 1.
Total number of first-degree relatives and number of screened relatives of 154 probands with definite aSAH.
| Total proband’s relatives (n) | Screened family members (n, %) | |
|---|---|---|
| Children | 298 | 144 (48) |
| Siblings | 499 | 181 (36) |
| Parents | 308 | 1 (0) |
| Total | 1105 | 326 (30) |
aSAH: aneurysmal subarachnoid haemorrhage.
Siblings of aSAH patients had a 1.62 (95% CI: 1.12–2.38) times higher risk of aSAH than children. Parents had a 0.44 (95% CI: 0.24–0.81) times lower risk than children (Table 2).
Table 2.
Number of aSAH in first-degree relatives of 154 index patients with aSAH.
| aSAH (n, %) | No aSAH (n, %) | Total proband’s relatives | RR (95% CI) | |
|---|---|---|---|---|
| Children | 35 (12) | 263 (78) | 298 | Reference |
| Siblings | 95 (19) | 404 (81) | 499 | 1.62 (1.12–2.38) |
| Parents | 16 (5) | 292 (95) | 308 | 0.44 (0.24–0.81) |
| Total | 146 (13) | 959 (87) | 1105 |
aSAH; aneurysmal subarachnoid haemorrhage; RR: relative risk; CI: confidence interval.
Siblings of aSAH patients had a 2.28 (95% CI: 1.23–4.07) times higher risk of UIA than children. When adjusted for age, the RR was 2.04 (95% CI: 1.07–3.92) (Table 3). The age at first screening was 40 years in children, 54 in parents and 55 in siblings. Because of the small numbers, we were not able to compare the risk of UIA in parents.
Table 3.
Number of UIA in screened first-degree relatives of index patients with aneurysmal subarachnoid haemorrhage.
| UIA (n, %) | No UIA (n, %) | Screened family members (n, %) | RR (95% CI) | RR adjusteda (95% CI) | |
|---|---|---|---|---|---|
| Children | 16 (11) | 128 (89) | 144 | Reference | |
| Siblings | 46 (25) | 135 (75) | 181 | 2.28 (1.23–4.07) | 2.04 (1.07–3.92) |
| Parents | 1 (100) | 0 | 1 | NR | |
| Total | 63 (19) | 263 (81) | 326 |
UIA: unruptured intracranial aneurysms; RR: relative risk; CI: confidence interval.
aAdjusted for age.
Discussion
Siblings of patients with familial aSAH have a significantly higher risk of both UIA and aSAH than children, and parents of patients with familial aSAH have a lower risk of aSAH compared to children. Analysis on the risk of UIA in parents compared to children was not possible because of the small number of parents screened for UIA.
Our results are in line with the findings of previous studies. In these studies, the risk of UIA and aSAH in siblings was in general somewhat higher than in our study, but CIs in these studies were wide. Our study is the largest study so far, and we found statistically significant results in contrast to some previous smaller studies. In a Dutch prospective screening study on UIA in 626 relatives of 160 aSAH patients, siblings had a four times higher risk of UIA than children.3 Another Finnish screening study in 837 relatives of 91 families with two or more affected members also found that the most common affected kinship were siblings.6 Furthermore, a community-based study from the US on 608 first-degree relatives of 81 aSAH patients found a higher ratio of the total observed cases with aSAH to the total expected in siblings in comparison to children and parents.7 One Swedish population-based case–control study on the risk of aSAH showed that type of kinship did not influence the risk on aSAH for individuals with one or more affected relatives. However, in this study, a population-based registry was used, in which case verification was conducted less strictly which may explain the differences in results.12
It is not clear why UIA and aSAH are more common in siblings as opposed to children and parents. UIA and aSAH are complex disorders which are caused by a complex interplay of multiple genetic and environmental risk factors.13 A higher burden of UIA and aSAH in siblings may suggest that there is a greater sharing of environmental risk factors between siblings than between children and parents. For example, previous studies have already shown that shared environmental effects on cardiovascular risk factors, including hypertension which is an important risk factor for both UIA and aSAH,14,15 are stronger for sibling pairs than for parent–offspring pairs.16 Generally additive effects of multiple genetic risk factors contribute to a complex disorder which would lead to comparative risks independent of family relationship. As an alternative explanation for the higher risk in siblings, it may be suggested that non-additive genetic effects are also involved.
The strength of our study is the large number of families included and the standardised screening protocol in our centre. There are also some limitations that need to be addressed. First of all, the children analysed were overall younger than the parents and siblings consequently might not have developed an UIA or aSAH yet. However, we do not think this has influenced our results, as when we adjusted for age in our analysis on UIA, the established higher risk in siblings compared to children remained essentially the same. We were not able to correct for age in our analysis on aSAH, because these data were frequently missing. Yet, siblings had an even higher risk on aSAH than parents, while parents were older and had more time to develop aSAH than siblings. Secondly, selection bias could have occurred. Probands or relatives of probands might not be well informed about their own family history, which may result in missing relatives who have had aSAH. Additionally, not all relatives who qualified to participate in the familial screening programme actually consented to undergo screening, and some relatives performed screening in another hospital than the UMC Utrecht.
Conclusion
Our study shows that siblings have an increased risk of UIA and aSAH compared to children. The type of kinship is a relevant factor to consider in the risk prediction and screening advice in families with familial aSAH.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: We acknowledge the support of The Netherlands CardioVascular Research Initiative: The Dutch Heart Foundation (CVON 2015–008 ERASE), Dutch Federation of University Medical Centers, The Netherlands Organization for Health Research and Development and the Royal Netherlands Academy of Sciences.
Ethical approval
Ethical approval for this study was obtained from Research Ethics Board of the University Medical Center Utrecht.
Informed consent
Informed consent was not sought for the present study because of the retrospective design.
Guarantor
YMR.
Contributorship
CZ researched literature, collected and analysed the data and wrote the first draft of the manuscript. YR and JG were involved in data analysis. All authors reviewed and edited the manuscript and approved the final version of the manuscript.
References
- 1.Vlak MH, Algra A, Brandenburg R, et al. Prevalence of unruptured intracranial aneurysms, with emphasis on sex, age, comorbidity, country, and time period: a systematic review and meta-analysis. Lancet Neurol 2011; 10: 626–636. [DOI] [PubMed] [Google Scholar]
- 2.Van Gijn J, Kerr RS, Rinkel GJ. Subarachnoid haemorrhage. Lancet 2007; 369: 306–318. [DOI] [PubMed] [Google Scholar]
- 3.Raaymakers TW. Aneurysms in relatives of patients with subarachnoid hemorrhage: frequency and risk factors. MARS Study Group. Magnetic resonance angiography in relatives of patients with subarachnoid hemorrhage. Neurology 1999; 53: 982–988. [DOI] [PubMed] [Google Scholar]
- 4.Teasdale GM, Wardlaw JM, White PM, et al. The familial risk of subarachnoid haemorrhage. Brain 2005; 128: 1677–1685. [DOI] [PubMed] [Google Scholar]
- 5.Bor AS, Rinkel GJ, van Norden J, et al. Long-term, serial screening for intracranial aneurysms in individuals with a family history of aneurysmal subarachnoid haemorrhage: a cohort study. Lancet Neurol 2014; 13: 385–392. [DOI] [PubMed] [Google Scholar]
- 6.Ronkainen A, Hernesniemi J, Puranen M, et al. Familial intracranial aneurysms. Lancet 1997; 349: 380–384. [DOI] [PubMed] [Google Scholar]
- 7.Schievink WI, Schaid DJ, Michels VV, et al. Familial aneurysmal subarachnoid hemorrhage: a community-based study. J Neurosurg 1995; 83: 426–429. [DOI] [PubMed] [Google Scholar]
- 8.Sundquist J, Li X, Sundquist K, et al. Risks of subarachnoid hemorrhage in siblings: a nationwide epidemiological study from Sweden. Neuroepidemiology 2007; 29: 178–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raaymakers TW, Rinkel GJ, Ramos LM. Initial and follow-up screening for aneurysms in families with familial subarachnoid hemorrhage. Neurology 1998; 51: 1125–1130. [DOI] [PubMed] [Google Scholar]
- 10.Schievink WI, Schaid DJ, Rogers HM, et al. On the inheritance of intracranial aneurysms. Stroke 1994; 25: 2028–2037. [DOI] [PubMed] [Google Scholar]
- 11.Wardlaw JM, White PM. The detection and management of unruptured intracranial aneurysms. Brain 2000; 123: 205–221. [DOI] [PubMed] [Google Scholar]
- 12.Bor AS, Rinkel GJ, Adami J, et al. Risk of subarachnoid haemorrhage according to number of affected relatives: a population-based case-control study. Brain 2008; 131: 2662–2665. [DOI] [PubMed] [Google Scholar]
- 13.Zhou S, Dion PA, Rouleau GA. Genetics of intracranial aneurysms. Stroke 2018; 49: 780–787. [DOI] [PubMed] [Google Scholar]
- 14.Feigin VL, Rinkel GJ, Lawes CM, et al. Risk factors for subarachnoid hemorrhage: an updated systematic review of epidemiological studies. Stroke 2005; 36: 2773–2780. [DOI] [PubMed] [Google Scholar]
- 15.Vlak MH, Rinkel GJ, Greebe P, et al. Independent risk factors for intracranial aneurysms and their joint effect: a case-control study. Stroke 2013; 44: 984–987. [DOI] [PubMed] [Google Scholar]
- 16.Harrap SB, Stebbing M, Hopper JL, et al. Familial patterns of covariation for cardiovascular risk factors in adults: the Victorian Family Heart Study. Am J Epidemiol 2000; 152: 704. [DOI] [PubMed] [Google Scholar]