Supplementary Fig. S1.
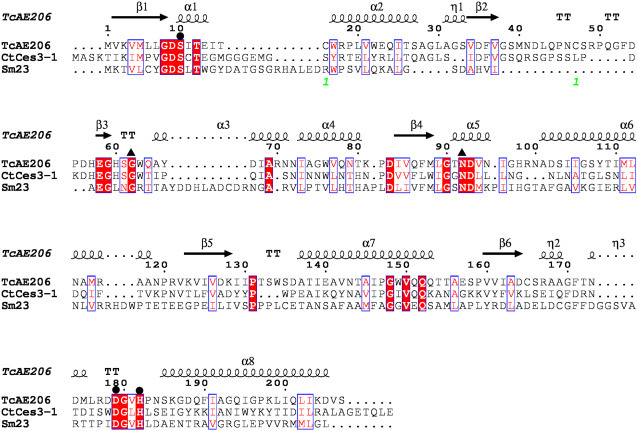
Structure-based multiple sequence alignment of CE3 esterases. Strictly conserved residues are boxed in red. Similar residues are written with red characters in blue boxes. Residues constituting the catalytic triad and oxyanion hole are indicated by black circles and black triangles, respectively. The α-helices (α1–8) and β-strands (β1–6) in TcAE206 are represented as black coils and black arrows, respectively. The two cysteine residues that form a S—S bond in TcAE206 are indicated by a light-green “1”. The sequence of the acetyl esterase from T. cellulolyticus (TcAE206; GenBank accession number BAP90856: the catalytic domain only) is aligned with that of the acetylxylan esterase from C. thermocellum (CtCes3-1; ABN52033) and that of the acetyl esterase from S. meliloti (Sm23; WP_003535461.1). The figure was made using ClustalW [29] and ESPript [30].
