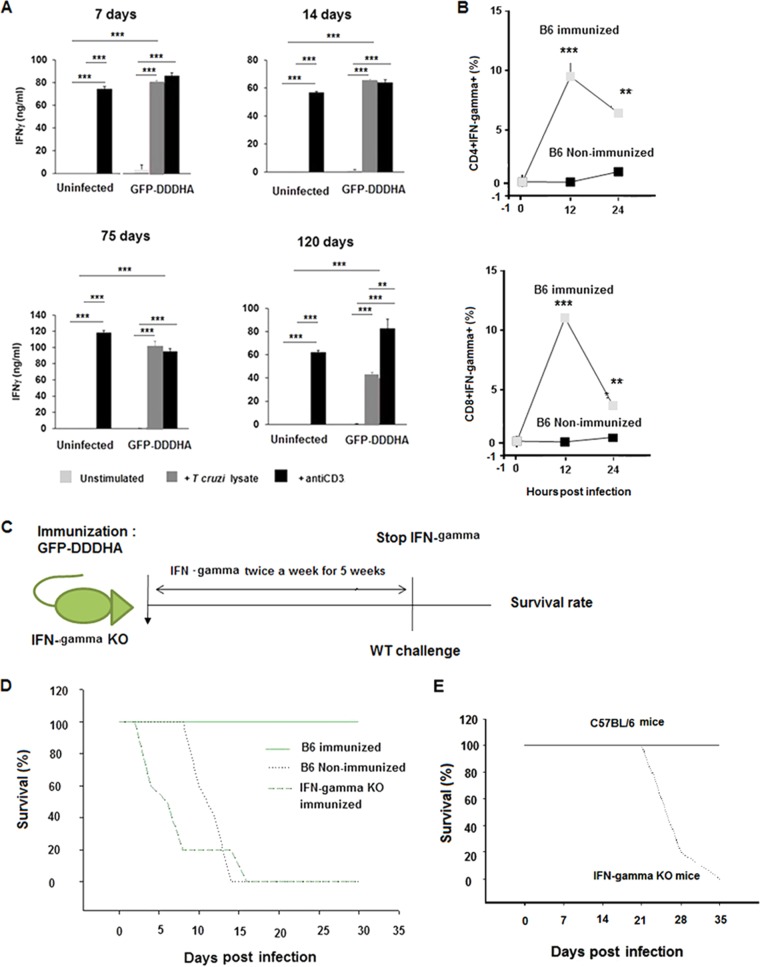
FIG 5.
Immunization with the GFP-DDDHA strain enhances IFN-γ expression during antigen reencounter. (A) C57BL/6 mice were inoculated with the GFP-DDDHA Tulahuen strain and sacrificed at the indicated time points. Single-cell suspensions of splenocytes were then left unstimulated or were stimulated with T. cruzi lysates or anti-CD3 antibodies. IFN-γ production was measured by enzyme-linked immunosorbent assay (ELISA). Graphs represent the average ± SEM of data obtained from 3 mice per time point and condition. Data were analyzed by ANOVA with Tukey’s posttest (days 7 to 14) or unpaired two-tailed t test (days 75 to 120). In summary, all uninfected cells stimulated with antibodies were significantly different (P < 0.001) from resting cells or cells stimulated from lysates, whereas in GFP-DDDHA strain-infected mice no differences were found between cells stimulated with lysates or with antibodies (except at day 120; P < 0.01), and both of them are different from the resting condition (P < 0.001) at any time point. All GFP-DDDHA strain-infected cells stimulated with lysates were different from uninfected cells stimulated with lysates (P < 0.001). **, P < 0.01; ***, P < 0.001. Data presented are one representative example of three separate experiments. All three experiments produced similar results. (B) Immunized C57BL/6 mice at 42 days postimmunization were reinfected with half a million WT Tulahuen parasites at 12 and 24 h postinfection. Splenocytes were analyzed by FACS and gated on CD4+ and CD8+ T cells, and the percentage of cells expressing IFN-γ was determined. Note that CD4+ and CD8+ T cells from immunized mice expressed much higher IFN-γ levels than those of nonimmunized mice. Data were analyzed with an unpaired two-tailed t test. **, P < 0.01; ***, P < 0.001. Data presented are one representative example of three separate experiments. All three experiments produced similar results. (C) Schematic representation of IFN-γ knockout mouse immunization, treatment with recombinant IFN-γ, and then challenge with WT parasites. Eight-week-old IFN-γ knockout mice and age-matched C57BL/6 mice were immunized with the GFP-DDDHA strain and then challenged with a WT infection. To allow protection to establish and to specifically analyze the role of IFN-γ in the secondary response to lethal infection, IFN-γ KO mice were administered recombinant IFN-γ (1.2 μg [1,000 U] per /mouse) twice weekly i.p. for 5 weeks starting 1 day postadministration of the GFP-DDDHA strain. After 5 weeks, treatment with IFN-γ was stopped, and the next day, following IFN-γ removal, the immunized IFN-γ-treated IFN-γ KO mice and the immunized C57BL/6 mice, as well as a group of nonimmunized C57BL/6 mice, were infected with 5 × 105 wild-type Tulahuen strain parasites. (D) Survival curve of IFN-γ knockout mice and control mice. Nonimmunized C57BL/6 mice and the immunized treated IFN-γ KO mice died with this lethal infection by day 17, while the immunized C57BL/6 mice all survived. This suggests that IFN-γ is important in mediating protection after immunization. Data presented are one representative example of two separate experiments. Both experiments produced similar results. (E) IFN-γ knockout mice died during GFP-DDDHA strain infection. Eight-week-old IFN-γ knockout mice were infected with 5 × 103 GFP-DDDHA Tulahuen strain parasites and received TMP-lactate treatment on day 7 postinfection. All mice died within 35 days postinfection, indicating that IFN-γ is essential for the development of immunity against T. cruzi (n = 10).