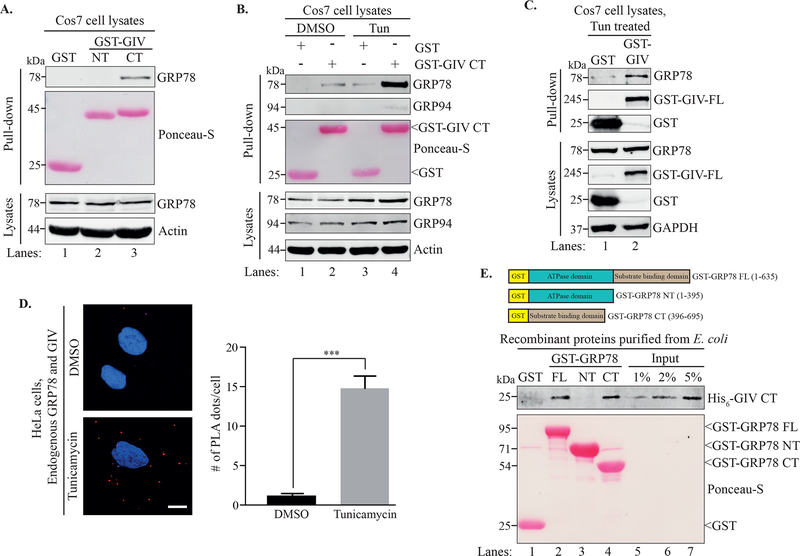
Figure 1. GRP78 and GIV interact in an ER stress-dependent manner.
A. A GST pull-down assay was performed using lysates of Cos7 cells expressing GST, GST-GIV-NT or GST-GIVCT. B. Cos7 cells expressing GST or GST-GIV-CT were treated with either DMSO or tunicamycin for 6 h before a GST pull-down was performed. Ponceau S staining shows the GST-tagged proteins from the pull-downs (A, B). C. Cos7 cells expressing either GST or GST-GIVFL were treated with tunicamycin followed by lysis and a GST pull-down. The pull-downs (A-C) were analyzed for binding to GRP78 and the immunoblots of the lysates confirm expression of the indicated proteins. D. HeLa cells treated with either DMSO or tunicamycin were fixed and analyzed for in situ interaction between endogenous GIV and GRP78 by performing a proximity ligation assay (PLA). The PLA dots (red) per cell were quantified from a total of 25–30 cells per experiment (right) from 3 independent experiments. Data are presented as mean ± S.E.M. ***p <0.001. Scale bar = 10 μm. E. Top. The schematic shows the different GST-tagged GRP78 constructs used for the experiment. Bottom. Equimolar amounts (0.2 μM) of bacterially expressed and purified GST-GRP78 protein constructs immobilized on glutathione agarose beads were incubated with purified His6-GIV-CT overnight. The pull-down samples were analyzed for His6-GIV-CT binding. Lanes 5–7 show the % input control for His6-GIV-CT used in the binding. Ponceau S staining shows GST-GRP78 constructs.