Abstract
Whole genome sequencing was performed on 20 matched blood and aqueous samples; tumor-associated chromosomal changes were found in 0/20 blood vs. 11/20 aqueous samples along with shorter DNA fragments. Thus, aqueous is superior to blood as a liquid biopsy for retinoblastoma.
Liquid biopsies utilize biofluid, often blood, to evaluate for tumor-derived cells or cell-free DNA (cfDNA) and have shown potential as a source of diagnostic and prognostic biomarkers. Unlike other cancers, retinoblastoma cannot be directly biopsied without risk of tumor seeding and spread outside the eye, making a liquid biopsy especially critical.1 We previously demonstrated that aqueous humor (AH) can be safely extracted from retinoblastoma eyes, that tumor-derived cfDNA is present in AH, and that genomic analyses of cfDNA reproducibly reflected somatic copy number alteration (SCNA) profiles of corresponding tumors.2 Additionally, we defined a tumor biomarker, 6p gain, as an indicator of aggressive disease.2 Gerrish et al. recently confirmed that both somatic RB1 mutations can be isolated from tumor DNA in the AH.3
AH extraction is minimally invasive and frequently indicated, including during intravitreal injection of chemotherapy for retinoblastoma. However, it involves insertion of a small-gauge needle through the clear cornea and entails a small risk of bleeding, infection, cataract, iris trauma and hypothetically, orbital seeding. The question remains whether blood, which is less invasive to extract than AH, also harbors retinoblastoma cfDNA.4 Thus, we evaluated whether blood demonstrates similar potential to AH as a liquid biopsy and source of detectable tumor-derived cfDNA for retinoblastoma (RB).
This analysis included patients diagnosed with retinoblastoma from August 2018 to February 2019. AH was taken at diagnosis or immediately before intravitreal injection of melphalan (IVM); matched blood samples were taken concurrently. Therapy was per CHLA protocol. AH sample processing was described previously.2 Methods for AH extraction, sequencing, bioinformatic and statistical analysis have also been described2 and are available at www.aaojournal.org. The primary endpoint was identification of tumor-derived cfDNA in AH and blood. Institutional Review Board/Ethics Committee approval was obtained.
Blood was collected via venipuncture and samples stored at room temperature in disodium EDTA collection tubes (BD, Franklin Lakes, NJ); processing was done within 72 hours. Samples underwent centrifugation for 10 min at 2000g. Clear plasma was collected and centrifuged at 14000g; the 2× cleared plasma supernatant was collected, and 100ul was used for cfDNA extraction as described for AH.2
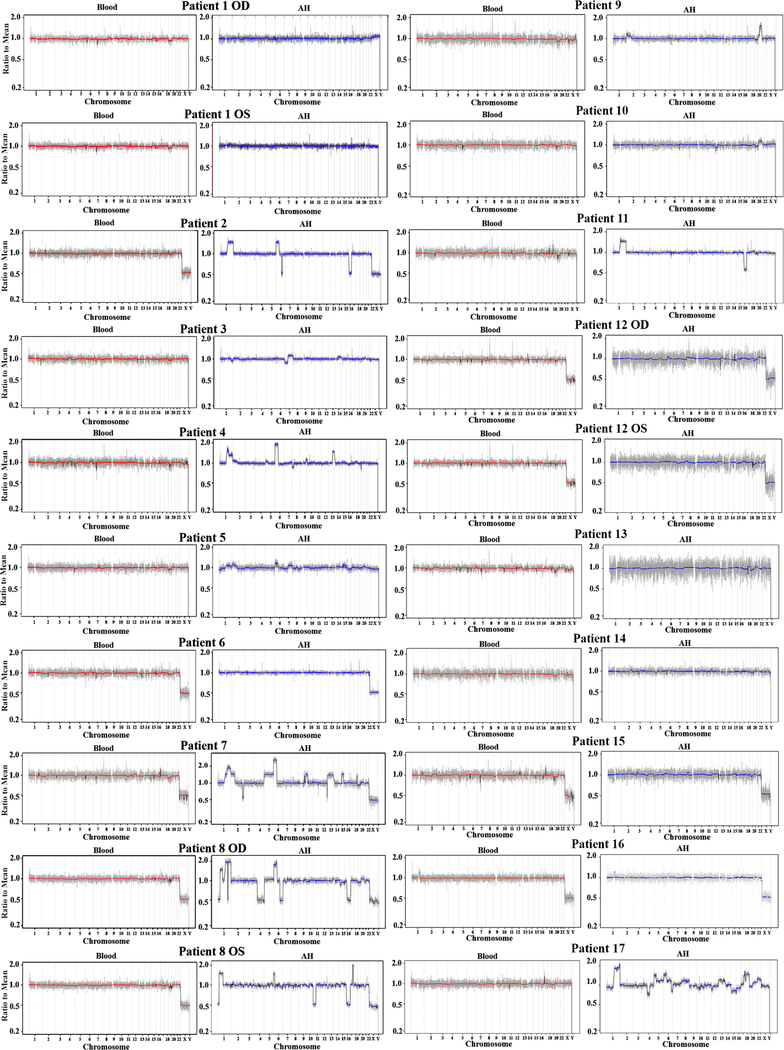
Twenty eyes of 17 patients were analyzed. Patient demographics are shown in supplemental Table 1 (available at www.aaojournal.org). Genome-wide SCNA profiles from AH samples (7 extracted at diagnosis, 13 during treatment) and matched blood samples were evaluated (Figure 1). Profiles consistent with RB5 were detected in 11/20 AH samples and 0/20 blood samples (p=<0.001, Fisher exact test). Of 7 AH samples extracted at diagnosis, 4 showed SCNAs (57%) while 7 of the 13 AH samples extracted during treatment demonstrated SCNAs (54%) (p=1.000). Median peak fragment size of cfDNA obtained from AH was significantly smaller than cfDNA obtained from serum (157.5 bp +/−10.6 bp in AH vs. 181.5 bp +/−3.2 bp in serum, p=<0.001).
Figure 1:
Genomic Profiles
Aqueous Humor (blue) and blood (red) genomic profiles from 20 eyes of 17 patients with retinoblastoma. No significant somatic copy number alteration (SCNA) relative to the median was found in the blood of any patient, however retinoblastoma associated SCNAs are seen in the cfDNA from the AH in patients 2,3,4,5,7, 8 OD and 8 OS, 9, 10, 11 and 17. Previous work4,6 has demonstrated that the chromosomal alterations seen in the AH are concordant with the changes in the retinoblastoma tumor (see also Figure 2, available at www.aaojournal.org). AH profiles from patients 8, 12, 13, 17 have been published previously (Berry et al. Mol Cancer Res 2018).
Herein we present analysis of retinoblastoma DNA in 20 separate AH and matched blood samples. We demonstrated both by size distribution and presence of SCNAs that AH appears to be a higher-yield source of cell-free tumor DNA than blood. Tumor-derived SCNAs were identified in AH only; these chromosomal alterations have been well-described in retinoblastoma as a mechanism for tumorigenesis.5 The smaller fragment sizes also indicate a tumor origin of AH cfDNA, as previous reports demonstrated shorter fragment size of tumor-derived circulating cfDNA (~150bp) compared to non-tumor origin.6 This adds to the Gerrish et al.3 report that the average size of cfDNA fragments in AH of retinoblastoma eyes are shorter than in plasma (average peak size 133bp vs 167bp).
Detection of circulating cell-free tumor DNA has led to a promising, active area of translational research in oncology. A liquid biopsy approach overcomes many difficulties and risks associated with obtaining traditional tissue biopsies and is especially critical in cancers like retinoblastoma wherein direct tissue biopsy is strictly contraindicated. However, liquid biopsies have their own inherent limitations, particularly for retinoblastoma. First, the suboptimal accuracy of tumor-DNA detection in blood is directly related to tumor burden. The concentration of DNA in AH also relates to tumor burden, with lower concentrations in treated eyes.2, 3 The comparatively small overall tumor burden of retinoblastoma, and its compartmentalization within the eye prior to metastatic spread, limits detection of circulating tumor DNA from blood. Deeper whole-genome sequencing may detect tumor DNA fraction down to 1% in blood (or AH), however the cost and time needed for these analyses limit the clinical utility of this approach. Another complicating factor is that in 1/3 of cases, retinoblastoma affects both eyes. A goal of liquid biopsy platforms is to provide prognostic information and follow for tumoral evolution over time. Even if improved sequencing modalities could detect circulating tumor DNA or other biomarkers in blood, it would be unclear how to assign prognosis in a bilateral case wherein the tumors in each eye likely have different profiles. Thus, the higher relative concentration of cfDNA in AH versus blood (percentage of tumor DNA to total volume), and the tumoral proximity for bilateral cases, continues to favor AH over blood as a liquid source of cell-free tumor DNA for retinoblastoma.
This preliminary work supports the utilization of AH as a liquid biopsy for retinoblastoma. Several studies have now demonstrated that AH is the highest yield liquid biopsy source of tumor-derived cfDNA and other biomarkers for retinoblastoma making it the highest potential biofluid for diagnosis, prognosis and monitoring treatment.2, 3 The cfDNA extracted from AH is representative of the genomic state of tumors2 (Figure 2, available at www.aaojournal.org)., can be assayed over time to correlate with tumor response to therapy2 and can be used instead of tumor tissue for detection of somatic RB1 tumor suppressor mutations.3 While extraction of AH is more invasive than a simple venipuncture, the procedure has been shown to be safe in the hands of a trained ocular oncologist.7 The direct comparative analysis in this sample set provides further support for the clinical utility of AH over blood as a source of tumor-derived cfDNA. Thus, there is merit in developing AH as a liquid biopsy for retinoblastoma diagnosis with further prognostic and therapeutic implications.
Supplementary Material
Acknowledgements
Research support from:
National Cancer Institute of the National Institute of Health Award Number K08CA232344 #IRG-16–181-57 from the American Cancer Society.
Wright Foundation
Knights Templar Eye Foundation
The Larry and Celia Moh Foundation
The Institute for Families, Inc., Children’s Hospital Los Angeles
An unrestricted departmental grant from Research to Prevent Blindness
Vicky Joseph Research Fund
Carol Vassiliadis Research Fund
USC Dornsife College of Letters, Arts and Sciences
Research Support:
National Cancer Institute of the National Institute of Health Award Number K08CA232344 #IRG-16–181-57 from the American Cancer Society.
Wright Foundation
Knights Templar Eye Foundation
Hyundai Hope on Wheels
The Larry and Celia Moh Foundation
The Institute for Families, Inc., Children’s Hospital Los Angeles
An unrestricted departmental grant from Research to Prevent Blindness
The National Institute of Health P30EY029220
Vicky Joseph Research Fund
Carol Vassiliadis Research Fund
USC Dornsife College of Letters, Arts and Sciences
Presentations: American Academy of Ophthalmology, accepted for ePoster discussion panel
Appendix: References
- 1.Karcioglu ZA, Gordon RA, Karcioglu GL. Tumor seeding in ocular fine needle aspiration biopsy. Ophthalmology 1985;92(12): 1763–7. [DOI] [PubMed] [Google Scholar]
- 2.Berry JL, Xu L, Kooi I, et al. Genomic cfDNA Analysis of Aqueous Humor in Retinoblastoma Predicts Eye Salvage: The Surrogate Tumor Biopsy for Retinoblastoma. Mol Cancer Res 2018;16:1701–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gerrish A, Stone E, Clokie S, et al. Non-invasive diagnosis of retinoblastoma using cell-free DNA from aqueous humour. Br J Ophthalmol 2019. doi: 10.1136/bjoophthalmol-2018-313005. epublished ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Uner OE, Ulrich BC, Hubbard GB. Potential of Aqueous Humor as a Surrogate Tumor Biopsy for Retinoblastoma. JAMA Ophthalmol 2018; 136(5):597–8. [DOI] [PubMed] [Google Scholar]
- 5.Kooi IE, Mol BM, Massink MP, et al. Somatic genomic alterations in retinoblastoma beyond RB1 are rare and limited to copy number changes. Sci Rep 2016;6:25264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mouliere F, Piskorz AM, Chandrananda D, et al. Selecting short DNA fragments in plasma improves detection of circulating tumour DNA. bioRxiv 134437 2017; doi: 10.1101/134437. [DOI] [Google Scholar]
- 7.Francis JH, Abramson DH, Ji X, et al. Risk of Extraocular Extension in Eyes With Retinoblastoma Receiving Intravitreous Chemotherapy. JAMA Ophthalmol 2017;135:1426–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.