Figure 3: Yck2 is the proximal target of GW responsible for restoration of echinocandin sensitivity in C. albicans.
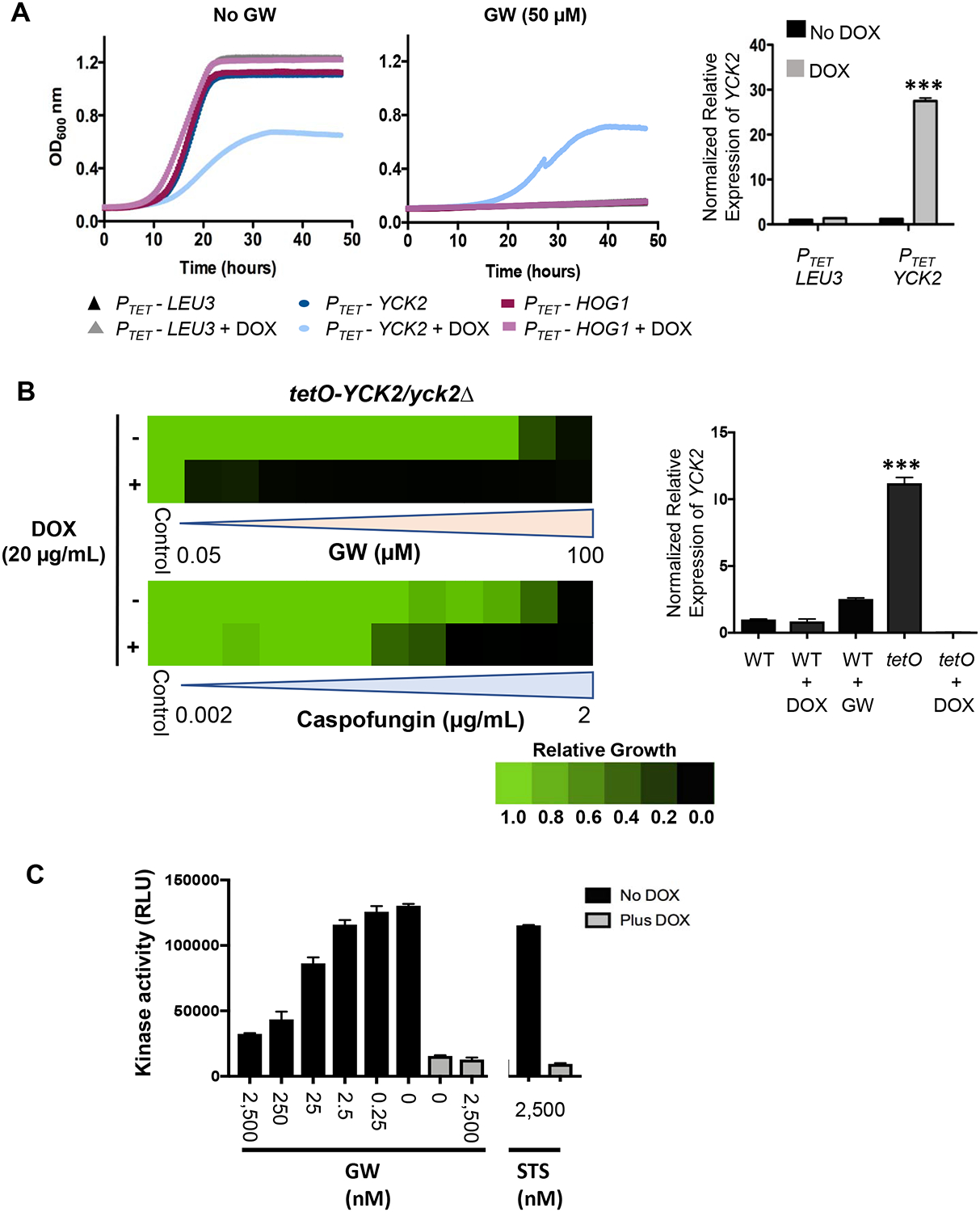
A, Overexpression of YCK2 confers resistance to GW-mediated growth inhibition. Recombinant strains transduced with the indicated genes under transcriptional control of a doxycycline (DOX)-inducible promoter (PTET) were grown in YPD medium in the presence or absence of GW (50 μM) and DOX (50 μg/mL) for 48 hours at 30°C. Growth was monitored every 15 minutes by absorbance at 600 nm (colored traces, left panels). The PTET-LEU3 strain served as a control for non-specific effects on growth. Each trace is the average of technical duplicates. Relative level of YCK2 overexpression induced by DOX treatment in the PTET-YCK2 strain was measured by quantitative reverse transcriptase PCR (qRT-PCR) and normalized to levels of GPD1. Bar graph (right panel) depicts the mean of triplicate samples. Error bars, SEM. DOX compared to no DOX treatment for PTET-YCK2 strain ***p <0.001 (unpaired student t-test) B, Inhibition of YCK2 expression sensitizes C. albicans to both GW and caspofungin. A recombinant strain in which one copy of YCK2 had been deleted and the other copy placed under transcriptional control of a DOX-repressible promoter (tetO-YCK2/yck2Δ) was grown in YPD medium for 48 hours at 30°C in the presence or absence of DOX (20 μg/mL) and serial dilutions of either GW (left, upper) or caspofungin (left, lower). Relative growth inhibition is displayed in heat-map format as described for Figure 1. Each colored box represents the mean of duplicate determinations. Color scale for relative growth is provided. Relative level of YCK2 expression in wild-type (WT, SN95) or tetO-YCK2/yck2Δ strain was measured by qRT-PCR and normalized to levels of GPD1 (right panel). Bar graph depicts the mean of triplicate samples. Error bars, SEM. WT cultured in GW (6 μM) compared to tetO-YCK2/yck2Δ (tetO) ***p <0.001 (student t-test). C, Concentration-dependent inhibition of Yck2 kinase activity by GW. A strain expressing HA-tagged Yck2 under control of a DOX-repressible promoter (tetO-YCK2-HA/yck2Δ) strain was grown in YPD medium in the presence or absence of 20 μg/mL DOX to log-phase at 30°C. Protein was extracted under non-denaturing conditions and Yck2 affinity precipitated with anti-HA magnetic beads. Bead aliquots were then assayed for kinase activity in the presence of 25μM ATP and the indicated concentrations of GW or the broad-specificity kinase inhibitor staurosporine (STS). The mean of duplicate determinations is presented. Error bars, SEM. The experiment was repeated twice with quantitatively similar results. (See also Figure S3).
