Abstract
Liver cancers, majority of which are primary hepatocellular carcinoma (HCC), continue to be on the rise in the world. Furthermore, due to the lack of effective treatments, liver cancer ranks the 4th most common cause of male cancer deaths. Novel therapies are urgently needed. Over last few years, immunotherapies, especially the checkpoint blockades and adoptive cell therapies of engineered T cells, have demonstrated a great potential for treating malignant tumors including HCC. In this review, we summarize the current ongoing research of antigen-specific immunotherapies including cancer vaccines and adoptive cell therapies for HCC. We briefly discuss the HCC cancer vaccine and then focus on the antigen-specific T cells genetically engineered with the T cell receptor genes (TCRTs) and the chimeric antigen receptor genes (CARTs). We first review the current options of TCRTs and CARTs immunotherapies for HCC, and then analyze the factors and parameters that may help to improve the design of TCRTs and CARTs to enhance their antitumor efficacy and safety. Our goals are to render readers a panoramic view of the current stand of HCC immunotherapies and provide some strategies to design better TCRTs and CARTs to achieve more effective and durable antitumor effects.
1. Introduction
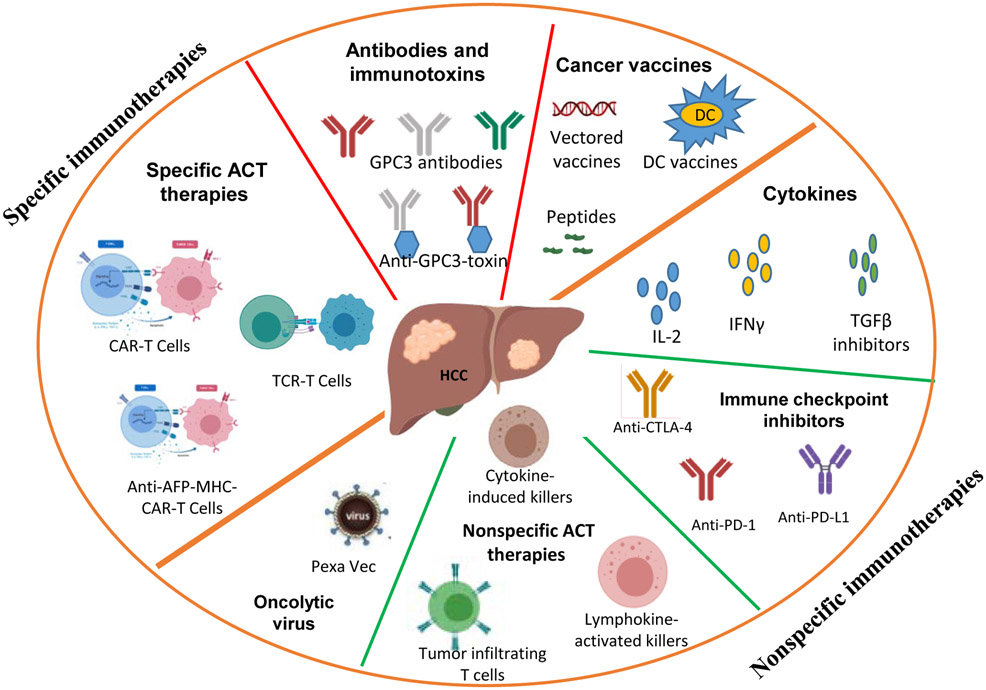
Liver cancer is the 6th most common cancer worldwide. Approximately 80-90% of the 854,000 annual cases are primary hepatocellular carcinoma (HCC) (Global Burden of Disease Cancer et al., 2017). While HCC caused by virus infection has leveled off thanks to HBV vaccination (Chang et al., 2009), HCC incidence due to obesity and other metabolic diseases has increased dramatically (El-Serag et al., 2004; Welzel et al., 2013). In fact, the incidence rate of liver cancer increases the fastest among all types of cancers in the United States. Unfortunately, treatments for liver cancer are limited and mostly ineffective. Although liver resection is curative, the lack of adjuvant therapy becomes a critical barrier to the success of surgery, which results in ~70% 5year recurrence rate (Kao et al., 2011) and ranks liver cancer the 4th most common cause of cancer death among male adults (Global Burden of Disease Cancer et al., 2017). Thus, novel therapies are urgently needed. Over the last few years, immunotherapy has demonstrated impressive efficacy for many tumors. Not surprisingly, multiple immunotherapeutic approaches are also being studied for HCC (Brar et al., 2018). Fig. 1 summarizes the current HCC immunotherapy studies. Broadly, immunotherapies can be divided into antigen-undefined (nonspecific) and antigen-defined (specific) immunotherapies. Nonspecific immunotherapies, including cytokines (such as IFNα, IL-2, TGF-β inhibitors, and so forth) and cytokines-induced killer cells (CIK) (Lee et al., 2015) have been studied in HCC for many decades, with some but uncertain antitumor efficacy. The HCC microenvironment is highly immune suppressive (Prieto et al., 2015) and overexpresses PD-L1 (Gao et al., 2009), the ligand for programmed death receptor 1 (PD-1). T cells in the tumor lesion are likely exhausted and overexpress exhaustion-related surface markers, such as PD-1, CTLA-4, TIM3, and LAG3 (Knolle and Thimme, 2014; Makarova-Rusher et al., 2015; Zhou et al., 2010). Immune checkpoint blockade (PD-1 and PD-L1 inhibitors) to rescue T cell function has generated impressive (~20%) overall response rate in treating late stage HCC patients (Hato et al., 2014), demonstrating the powerful therapeutic effects of activating the patient’s own immune system. On the front of specific immunotherapies, cancer vaccines, T cell receptor (TCR) and chimeric antigen receptor (CAR) geneengineered T cells (TCRTs and CARTs) have been actively studied for HCC. For a comprehensive view of the current HCC immunotherapies, including both the nonspecific and specific immunotherapies, please refer to the latest reviews (Buonaguro et al., 2019; Mizukoshi and Kaneko, 2019). In this review, we mainly focus on specific immunotherapies. We briefly summarize the current HCC cancer vaccines and then focus on reviewing the ongoing studies of TCRTs and CARTs in HCC immunotherapy. We also analyze the factors that may improve the antitumor efficacy and the safety parameters of engineered T cells.
Fig. 1.
Current immunotherapies for HCC. GPC3, Glypican3; ACT, adoptive cell therapy; DC, Dendritic cells; TCR, T cell receptor; CAR, Chimeric antigen receptor.
2. Cancer vaccines
Even though autologous dendritic cells (DCs) pulsed with antigen-undefined tumor cell lysate have also been explored to treat HCC (Palmer et al., 2009), it is the antigen-defined cancer vaccines that draw the interest of most researchers. Specific HCC cancer vaccines are derived from HCC-associated antigens, which mainly include alpha fetoprotein (AFP), glypican 3 (GPC3), New York esophageal squamous cell carcinoma-1 (NY-ESO-1) cancer testis antigen, and human telomerase reverse transcriptase (hTERT). Table 1 summarizes the HCC cancer vaccines. Overall, like all cancer vaccines, the antitumor efficacy of HCC cancer vaccines is limited and transient although some anecdotal antitumor effect was found. However, a recent study showed that one potential application of cancer vaccines is to expand the antigen specific engineered T cells after adoptive cell transfer (Ma et al., 2019).
Table 1.
Summary of the clinical trials of HCC cancer vaccines
| Antigen | MHC I | Epitope | IR detected | Antitumor response | Reference |
|---|---|---|---|---|---|
| AFP | A0201 | 137, 158, 325, 542 | 6/10 PTs | Not observed | Butterfield et al, 1999, 2001, and 2006 |
| A2402 | 357, 403, 414, 424, 434 | 5/15 PTs | 1 CR (>2yr); 8 SD (PFS 64 days); 6 PD | Mizukoshiet al, 2006 Nakagawa et al, 2017 | |
| GPC3 | A0201 | 144 | 13/16 PTs | 1PR, 19 SD (>2mos), 13 PD | Komori et al, 2006 Sawada et al, 2012 |
| A2402 | 298 | 17/17 PTs | |||
| hTERT | A2402 | 167, 324, 461, 637, 845, 1088 | 11/14 PTs | IR correlates to delay of local tumor recurrence | Mizukoshi et al, 2006 Mizukoshi et al, 2015 |
| NY-ESO1 | A0201 | 157 | Ongoing trial of NY-ESO1 pulsed dendritic cells, no data yet | ||
The epitope number indicates the position of the 1st amino acid of the epitopes (9 amino acids in length). Abbreviations are as follows; IR, immune responses; PT, patient; CR, complete response; SD, stable disease; PFS, progression free survival; PD, progression disease.
2.1. AFP:
AFP is an oncofetal protein that is expressed during the fetal stage and becomes undetectable after birth. AFP is highly re-expressed in HCC and its level is correlated to the severity of the disease. AFP has been used as an HCC tumor marker for many decades. As an immunotherapy target, it was first studied by Butterfield et al. who identified 4 HLA-A2-restricted AFP epitopes (Butterfield et al., 1999; Butterfield et al., 2001). These peptide-based vaccines did induce T cell responses in patients, but did not generate significant clinical benefits (Butterfield et al., 2006). One possible reason is the fact that high affinity T cells against self-antigen are deleted during thymus maturation. To overcome this problem, we developed an epitope-optimized genetic vaccine to activate the self/tumor antigen-specific T cells (Liu et al., 2009). We mutated the mouse AFP so that the potential epitopes would not be considered “self” and thus could activate high affinity T cells (Hong et al., 2014). Importantly, T cells activated by the epitope-optimized AFP were able to recognize and bind wild-type MHC/AFP complexes. Immunization with such epitope-optimized vaccines effectively prevented and treated carcinogen-induced mouse autochthonous HCC tumors (Hong et al., 2014). Unfortunately, we were unable to translate this finding and generate epitope-optimized human AFP cancer vaccines (unpublished data). In another study, Mizukoshi et al identified 4 HLA-A2402-restricted AFP epitopes, and immunization with the peptides induced low level T cell responses in HCC patients (Mizukoshi et al., 2006b). More promising data were obtained in a phase 1 trial (Nakagawa et al., 2017). Administration of these HLA-A2402- restricted AFP peptides (AFP357 and AFP403) to 15 patients with HCC did not cause adverse events and induced peptide-specific T cell responses. Importantly, complete response was observed in 1 patient and stable disease of 2 months was obtained in 8 patients. A highly functional TCR was expressed in T cells from a patient with complete response. Even though it is not clear whether large trials will be equally promising, this small scale study indicates that cancer vaccines could be effective.
2.2. Glypican 3 (GPC3):
GPC3 is a glycosylphosphatidylinositol (GPI)-anchored membrane protein. It is highly expressed in fetal liver but becomes undetectable after birth, and regains expression in the majority of HCC tumors (Filmus and Capurro, 2013; Nakatsura et al., 2004a). GPC3 is implicated in the WNT signaling pathway to promote proliferation of tumor cells and is associated with poor prognosis in HCC (Nakatsura et al., 2004a; Pez et al., 2013). In 2004, Nakatsura et al. reported that mouse GPC3 could activate T cell responses in mice (Nakatsura et al., 2004b). Then, they identified the HLA- A2- restricted GPC3144 epitope and the HLA-A24-restricted GPC3298 epitope in humans (Komori et al., 2006). These GPC3 vaccines generated some positive effects in clinical trials (Sawada et al., 2012). However, several more trials indicated that even though GPC3-specific immune responses are activated, the clinical benefits are minimal or uncertain in most advanced HCC patients (Shimizu et al., 2018).
2.3. NY-ESO-1:
Cancer-testis antigen NY-ESO-1 was found in many types of human cancers, including HCC (Luo et al., 2002). NY-ESO-1 cancer vaccines induced both humoral and cellular immune responses in cancer patients (Caballero and Chen, 2009; Gnjatic et al., 2006). NY-ESO-1157-specific T cells response was also reported in HCC patients (Korangy et al., 2004), especially after depletion of Tregs (Zhang et al., 2010). Dendritic cells loaded with multiple antigens including the NY-ESO-1 are being tested in clinical trials including HCC patients ( NCT03175705), but no data on the antitumor efficacy have been reported yet.
2.4. hTERT:
It has been reported that ~80% of HCCs express hTERT (Zhou et al., 2015). Mizukoshi et al. identified several A24-restricted hTERT epitopes (Mizukoshi et al., 2006a). These peptides could induce hTERT-specific T cell immune responses in HCC patients and the immune responses were correlated to the delay of tumor recurrence at local and distant site following radiofrequency ablation treatment (Mizukoshi et al., 2015).
2.5. Neoantigen vaccines:
Although the clinical outcome of cancer vaccines using tumor associated selfa-ntigens over the last 30 years has been disappointing, recent cancer vaccines based on neoantigens have generated some promising results. Tumor genome deep sequencing reveals that tumor masses can accumulate many mutations (Lawrence et al., 2013). High tumor mutation is associated with better immunotherapeutic outcomes (Rizvi et al., 2015). Such mutated proteins form the basis of neoantigen cancer vaccines. While T cells against shared self-antigens have low affinity and are ineffective in most cases, T cells against neoantigens are considered of high affinity and have better potential of generating clinical benefits. Recent studies have showed that such neoantigen-based vaccines can be successful in treating late stage cancers (Ott et al., 2017; Sahin et al., 2017). High throughput DNA sequencing revealed that HCC tumors also harbor many mutations, especially in the genes of p53, TERT, beta-catenin and WNT (Lee, 2015; Schulze et al., 2015; Yao et al., 2016; Zucman-Rossi et al., 2015). Like all other tumors, mutations in HCC are highly heterogenic and may require personalized vaccines. Although no neoantigen-based cancer vaccines have been developed for HCC yet, such studies are likely to be actively pursued.
3. TCRTs
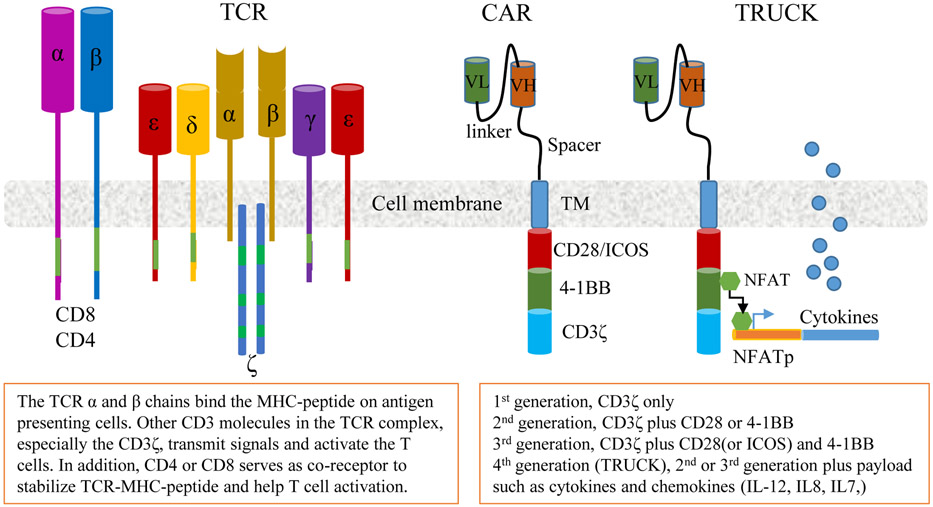
The T cell receptor (TCR) is a member of the immunoglobulin superfamily, consisting of the TCRα and TCRβ chains, which confer specificity to the receptor. The TCR complex also includes the CD3ε, CD3γ, CD3δ, and CD3ζ chains (Fig. 2) that play important roles in TCR signaling. T cells transduced with exogenous TCRs (TCRTs) can recognize both surface and intracellular antigens presented through major histocompatibility complexes (MHC). Thus the recognition and binding of the TCRs to tumor peptides are MHC-restricted. In contrast, chimeric antigen receptors (CARs), which are synthetic receptors, bind cell surface antigens without MHC restriction. CARs combine an antigen binding region derived from an antibody (or a receptor ligand) and the intracellular signaling domain of CD3ζ with one or more co-stimulatory signaling domains (Fig.2). Since ~90% of tumor antigens are intracellular proteins, TCRTs should have broader applications than CAR engineered T cells (CARTs). The binding affinity of CARs for surface antigens is higher than that of TCRs for binding MHC/peptide complexes. However, the advantages and disadvantages of high affinity binding CARs vs lower affinity binding TCRs is not known. In fact, CARTs have not proved to be effective in treating solid tumors yet. On the other hand, NY-ESO-1 specific TCRTs generated significant antitumor effects in treating multiple myeloma (Rapoport et al., 2015) and synovial sarcomas (D'Angelo et al., 2018; Robbins et al., 2015).
Fig. 2.
The schematic diagrams of TCR complex, the CAR, and the TRUCK. TCR, T cell receptor; CAR, Chimeric antigen receptor; VL, variable region of light chain of antibody; VH, variable region of heavy chain of antibody. TM, transmembrane domain.
3.1. Approaches to identify TCRs:
TCRs used for creating TCRTs can be derived from humans or mice. Several approaches are employed to identify antigen-specific TCRs. 1) Conventionally, tumor-specific human T cell clones are obtained in vitro through repeated antigen stimulation of human T cells, which may likely eliminate the high affinity T cells if some do escape thymus deletion. 2) Recently, single T cell sequencing allowed for the identification of multiple paired TCR genes (Kobayashi et al., 2013), but it required extensive work to verify their specificity and function afterwards. Normally, a person’s T cell repertoire has been selected in the thymus and the high affinity T cells against self-antigens are deleted. Thus, TCRs obtained from human T cell repertoires via the aforementioned approaches are of low affinity. Thus, mutations have been introduced into the CDR regions to enhance TCR affinity. 3) Another way to obtain high affinity human TCRs is to use allogenic T cell stimulation (Dargel et al., 2015). In this approach, HLA-A2 negative donor T cells and antigen presenting cells are used to identify high affinity TCRs targeting the HLA-A2/epitope. 4) High affinity human TCRs can also be identified from the human HLA and TCR repertoire double-humanized transgenic mice (Li et al., 2010; Obenaus et al., 2015). In these double transgenic mice, both the MHC and the TCR are human, but the T cell repertoire is not selected against human antigens, and should contain high affinity TCRs. However, the double- humanized mice have not been widely used. 5) We recently developed a prime-boost immunization strategy to identify human AFP-specific mouse TCRs (Zhu et al., 2018). The lentiviral vector can prime AFP-specific CD8 responses in HLA-A2 mice and the intravenous boost with particular peptide epitopes induce a large number of antigen-specific T cells, which allow us to study their antitumor function and identify multiple TCRs with high diversity. The TCRs from the HLA-A2 transgenic mice were naturally of high affinity.
3.2. HCC-specific TCRs:
TCRs targeting several HCC-associated tumor antigens have been identified, and studied, some of which are currently in clinical trials. A summary of current TCRs for HCC immunotherapy is listed in Table 2.
Table 2.
Summary of the TCRs for HCC associated antigens
| Antigen | TCR origin | HLA | Epitope | Reference | Ongoing trial | Antitumor effect |
|---|---|---|---|---|---|---|
| AFP | Mouse | A0201 | 158-166 | Zhu et al, 2018 | NCT03971747 | No report yet |
| Human | A0201 | 158-166 | Docta et al, 2019 | NCT03132792 | No report yet | |
| Human | A2402 | 357-365 | Nakagawa et al, 2017 | None recorded | ||
| GPC3 | Human | A0201 | 367-375 | Dargel et al, 2015 | None recorded | |
| HBsAg | Human | A0201 | 183-191, 370-378 | Gehring et al, 2011 Qasim et al, 2015 | NCT02686372 | No report yet |
| NY-ESO1 | Human | A0201 | 157-168 | Li et al, 2005 Robbins 2008 | NCT02869217, NCT03159585 | No report yet |
| Mouse | 157-168 | Rosati et al, 2014 | NCT01967823 | No report yet | ||
| hTERT | Human | A2402 | 461-469 | Mizukoshi et al, 2015 | None recorded |
The clinical trials of TCRTs for HCC are ongoing and no clinical outcome has been reported yet.
3.2.1. AFP-specific TCRs:
Several AFP-specific TCRs are identified and being tested in preclinical tumor models and in clinical trials.
HLA-A2/AFP158-specific mouse TCRs: Using the immunization strategy of recombinant lentiviral vector priming (He et al., 2005) and peptide boosting, we achieved a very high level of HLA-A2/AFP158-specific CD8 T cells in HLA-A2 transgenic mice (10-30% of CD8 T cells) (Zhu et al., 2018). The easy availability of such high numbers of AFP-specific T cells allowed us to study their antitumor effect in vitro and in vivo. Adoptive transfer of the HLA-A2/AFP158-specific CD8 T cells from the HLA-A2 transgenic mice resulted in complete regression of human HCC tumors as large as 2cm in diameter in immunocompromised NSG mice. From these HLA-A2/AFP158-specific CD8 T cells we then identified 3 TCRs that are naturally of high affinity. Human T cells engineered with these TCRs (TCRTs) generated potent antitumor effects in vitro and in vivo. Systematic X-Scan data showed that the three TCRTs have minimal or no cross-reactivity against human cells (unpublished data). A clinical trial using these TCRTs to treat HCC patients has been initiated ( NCT03971747).
HLA-A2/AFP158-specific human TCRs: Recently, Docta et al. reported the identification of a human TCR specific for the HLA-A2/AFP158 complex from a AFP-specific human T cell clone and affinity enhancement (Docta et al., 2019). They also conducted a systematic X-scan study and found no cross-reactivity with other peptides in the human protein databank. A clinical trial using patient’s autologous T cells engineered with this AFP-specific TCR has been initiated and is undergoing ( NCT03132792).
HLA-A24/AFP357-specific human TCRs: While the HLA-A2 serotype is very common among Caucasians, A24 is another subtype of HLA commonly found in Asians. Nakagawa et al. recently reported a high affinity TCR that recognizes the HLA-A2402/AFP357 complex (Nakagawa et al., 2017). This TCR was identified from a HCC patient with complete response (CR) after AFP peptide vaccination. Even though the killing activity against HepG2 tumor cells by the human TCRTs generated from the HLA-A2402/AFP357-specific TCRs was rather low, it nevertheless offers the potential of developing TCRTs for HCC patients with the HLA-A24 subtype.
3.2.2. GPC3-specific TCRs:
As already mentioned, GPC3 is another HCC-associated tumor antigen. In 2015, a German group identified anti-GPC3 human TCRs from an HLA-A2 negative donor (Dargel et al., 2015). In theory, the TCR repertoire in an HLA-A2 negative donor is not selected against HLA-A2 presented self-peptides. Thus, it should contain high affinity TCRs against HLA-A2/GPC3 peptides. They co-transfected the HLA-A2 negative donor’s dendritic cells with HLA-A2 molecules and GPC3 and used them as APCs to activate the donor’s own T cells. By doing so, they were able to identify HLA-A2/GPC3367 specific TCRs. But, it is not clear whether this TCR- derived allogenic human T cells are in clinical trials.
3.2.3. Virus-specific TCRs:
A lower percentage of HBV-infection derived HCC tissues maintain their HBV gene expression, which can become TCRT targets. A Singaporean group identified HBV surface antigen specific TCRs in the early 2010s (Gehring et al., 2011) which are currently in clinical trials (Qasim et al., 2015) ( NCT02686372). Similarly, another group developed an HCV antigen-specific TCR. These HCV-specific TCRTs regressed HCV+ HCC tumor xenografts in a mouse model (Spear et al., 2016).
3.2.4. NY-ESO-1157-specific TCRs:
The NY-ESO-1157 TCRs are the most studied TCRs. Both mouse derived TCR (Rosati et al., 2014) and human derived, and enhanced (Li et al., 2005; Robbins et al., 2008) TCRs have been identified. There are ~30 clinical trials using these TCRTs to treat a variety of cancers (Zhang and Wang, 2019). These TCRs have been very successful in treating multiple myeloma (Rapoport et al., 2015) and synovial sarcoma (D'Angelo et al., 2018; Robbins et al., 2015). Due to NY-ESO-1 overexpression, HCC is included in the ongoing trials testing the NY-ESO-1 TCRTs (Phase II: NCT01967823; Phase I: NCT02869217 and NCT03159585). But, it is not clear how many HCC patients were actually enrolled in the trials.
3.2.5. hTERT-specific TCRs:
In the study of hTERT peptide vaccination in HCC patients, Mizukoshi et al. also identified an hTERT461-specific TCR (Mizukoshi et al., 2015), which may have the potential of being used to create TCRTs for HCC immunotherapy.
3.3. TCRT safety:
Because of the promiscuity of the TCR, TCRTs may cross-react with off-target peptides. Over the last decades, several TCRTs have been tested in clinical trials, allowing investigators to evaluate their safety. While NY-ESO-1 TCRTs have been safe in human trials (Stadtmauer et al., 2019), other TCRTs generated significant off-target cardiac and neurological toxicity (Brichard et al., 2013; Linette et al., 2013). Both mouse and human TCRs can generate off-target cross-reactivity. In fact, the MAGE-A3/HLA-A1 TCR was derived from humans, but generated significant off-target cardiac toxicity via targeting heart muscle protein Titin (Cameron et al., 2013). Because of the incapability of using animal models to predict TCRT’s toxicity, a preclinical safety package has been developed to evaluate TCRT’s potential toxicity, which includes on-target/off-tumor and off-target toxicity, and alloreactivity with other unintended HLA allotypes. The TCRT’s off-target cross-reactivity can be studied by X-scan (Border et al., 2019), which is conducted by activating TCRTs with a panel of 171 X-peptides generated by replacing each amino acid residue with any other 19 amino acids. A peptide motif recognized by TCRTs will then be identified and used to search on protein databanks to find the actual peptide sequences. Next, the actual peptides from the databanks will be synthesized to test if they can indeed activate TCRTs. Finally, cells expressing the off-target protein harboring the reactive peptides will be tested to activate TCRTs. If the TCRTs are activated by the off-target expressing cells, the TCRTs will generate off-target cross-reactivity and should not be used for human trials. In some cases, the safety concern of TCRTs can be addressed by adding a safety tag to safely remove them if necessary. This will be further discussed in the section of CART’s safety.
3.4. TCRT improvements:
While NY-ESO-1 TCRTs demonstrated clinical antitumor efficacy, most of other TCRTs have yet to be proved effective in patients. Several factors can be considered to improve the antitumor efficacy of TCRTs, which include extension of the survival of TCRTs, improvement of homing, and prevention of T cell exhaustion. Because similar strategies will be used to improve CART’s efficacy, we will address this issue below, in the section of CARTs.
4. CARTs
Multiple CART clinical trials for HCC are undergoing around the world. Most of the trials target GPC3, while another trials target the MHC/AFP158 peptide complex. In this section, we will first review the current CART studies for HCC treatment, and then focus our effort on understanding the factors that may influence the functioning of CARTs. We propose the strategies to create more effective and safe CARTs for solid tumors, including HCC.
4.1. CART immunotherapies for HCC:
A number of CART immunotherapies are being developed for HCC (Table 3). They are mainly targeting GPC3 and AFP.
Table 3.
Summary of CARTs immunotherapies for HCC
| Target | scFv | Epitope | Generation of CARs |
ICD | References | Clinical trial |
|---|---|---|---|---|---|---|
| GPC3 | GC33 | GPC3524-563 | 2nd | 4-1BB CD3ζ | Jiang et al, 2016 Li et al, 2017 | NCT03130712, NCT03084380, NCT02715362, NCT02905188, |
| 3rd | CD28-BB-CD3ζ | Gao et al, 2014 | NCT02395250 | |||
| 4th | 2nd + IL15/IL21 | Alizadeh et al, 2019 Krenciute et al, 2017 | NCT04093648 | |||
| 2nd+ IL7/CCL19 | Adachi et al, 2018 | NCT03198546 | ||||
| HLA/AFP | ET1402L1 | A0201/AFP158 | 2nd | CD28-CD3ζ | Liu et al, 2017 | NCT03349255 |
| ET140202 | A0201/AFP158 | Ab-TCR mimics | γ δ TCR | Xu et al, 2018 | NCT03965546, NCT03998033 |
All the current CARTs targeting GPC3 are derived from monoclonal antibody GC33. There is no report of antitumor effect yet on the CART’s clinical trials. scFv, single chain fragment of variable region; ICD, intracellular domain; Ab-TCR, Fab fragment of antibody-TCR.
4.1.1. GPC3-specific CARTs:
The antibody variable region is a critical component of a CAR. A number of antibodies have been developed against human GPC3 (Feng and Ho, 2014). One of the antibodies, GC33 (binding to the membrane-proximal C-terminal epitope of GPC3) has advanced to clinical trials and has proved to be safe. However, it failed to generate significant antitumor effects (Abou-Alfa et al., 2016). Several groups then utilized the GC33 antibody to develop CARTs (Alizadeh et al., 2019; Gao et al., 2014; Jiang et al., 2016; Krenciute et al., 2017; Li et al., 2017b) and their antitumor effect is being investigated in clinical trials. In addition, CARTs derived from antibodies YP7 (also binding to membrane-proximal epitope) and HN3 (binding conformational epitope) are being studied (Li et al., 2018). To improve CART safety, Chen et al. recently developed GPC3-ASGR1 dual CARTs (Chen et al., 2017). Presumably, these dual CARTs will improve safety as they will kill tumor cells expressing both GPC3 and ASGR1 antigens. Furthermore, the dual CARTs persisted longer and generated stronger antitumor effects in animal models.
4.1.2. GPC3- CAR-NK:
In one study (Yu et al., 2018), the authors utilized NK cells as the host cells and created CAR-NK cells. In this study, a different GPC3 antibody (9F2) variable chain was used to build the CAR. It is not clear whether the CAR-NK cells would be more effective and safer than CARTs as no comparative study was done.
4.1.3. MHC/AFP158 complex-specific CARTs:
By far, most CARTs target cell surface antigens. However, 90% of tumor antigens are intracellular proteins. All antigens, regardless of location, can be loaded onto MHC molecules and be presented on the cell surface during their synthesis and degradation. Thus MHC/peptide complexes are accessible to CARs. But in this situation, the CAR binding to peptides will be MHC-restricted. Several of such CARs were designed and tested (Maruta et al., 2017; Rafiq et al., 2017). Liu et al explored this novel concept and developed an antibody (ET1402L1) against the HLA-A2/AFP158-166 peptide complex and then created CARTs, and demonstrated their antitumor efficacy in a preclinical study (Liu et al., 2017). A Phase 1 trial ( NCT03349255) is undergoing in China, and preliminary data showed no severe side effects. In addition, the Fab fragment of this antibody was fused with γδ TCR chain to develop antibody TCR mimics (Ab-TCR mimics) to take advantage of the natural TCR assembly and signaling pathway (Xu et al., 2018). Recently, two clinical trials based on this Ab-TCR mimics were initiated in China ( NCT03965546) and in USA ( NCT03998033) to study their safety and effectiveness in treating advanced HCC.
4.2. Parameters governing the antitumor efficacy of CARTs:
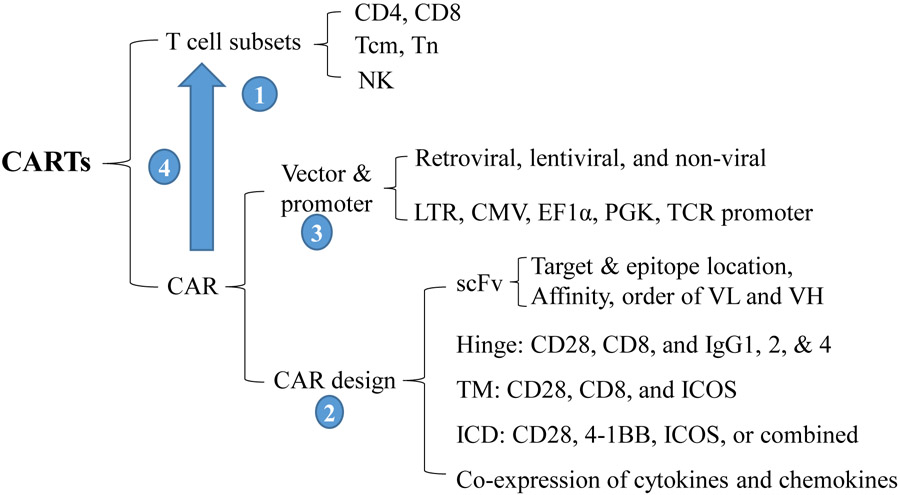
Several CART therapies have been successful for hematological cancers. However, many other CARTs, especially those for solid tumors, have not yet proved to be effective. Clearly, improvement of the efficacy of CARTs is needed. An ideally engineered CART should respond to and expand in an antigen-dependent manner without incurring in rapid T cell exhaustion. In the absence of antigen, CARTs should have “zero”, or very low, background activation (tonic signaling). In vivo, CARTs should expand with proper function in tumor lesion to generate antitumor effects, and be long-lasting to prevent tumor recurrence. Because CARTs are generated from transduction of T cells with CAR genes, the selection of the T cell subsets to be engineered, the design of CAR genes, the gene delivery vectors, and the production process will all affect the quality of the CARTs. Here, we review the parameters that affect CART’s efficacy (Yan and Liu, 2019). Some of these parameters may also be applicable to TCRTs.
4.2.1. T cell subsets:
Most CARTs are generated from using unfractionated T cells (Jensen and Riddell, 2014) composed of total CD4 and CD8 T cells at different activation and differentiation stages, which may vary significantly among different donors, especially for cancer patients undergoing heavy chemo and radiation treatments. Recent studies suggest that T cell subsets selected to be engineered with CARs could greatly affect the antitumor effect of the CARTs by contributing to T cell expansion, effector function and long-term persistence in vivo. Such defined T cell subsets may become particularly important for treating solid tumors, in which the response rate needs to be improved.
CD4 vs CD8: Using unfractionated total T cells, Louis et al. showed that higher initial percentage of CD4 T cells was associated with longer persistence of GD2-specific CARTs (Louis et al., 2011). Using isolated T cell subsets and CD19 CAR, one study showed that, compared to CD4+CARTs, CD8+CARTs exhibited higher lytic activity, weaker proliferation, and lower cytokine production in response to target tumor cell stimulation (Sommermeyer et al., 2016). The study also confirmed that CD4+CARTs survived longer than CD8+CARTs, even though CD8 CARTs were as good as CD4 CARTs in treating B cell leukemia in an animal model (Sommermeyer et al., 2016). In a different preclinical study, using an IL13Rα2-specific (IL13-41BB-CD3ζ) CAR for brain tumors, Wang et al showed that these glioblastoma-targeted CD4+CARTs outperformed CD8+CARTs (Wang et al., 2018). CD4+CARTs not only kill tumor cells directly, but also are essential for long-term antitumor efficacy, and could be less prone to activation-induced exhaustion and differentiation (Wang et al., 2018). Moreover, repetitive stimulation with tumor cells resulted in exhaustion of CD8+CARTs, but not of CD4+CARTs. Importantly, the mixture of CD4 and CD8 CARTs resulted in less antitumor effect than CD4+ CARTs alone. The antitumor effects of CD4 and CD8 CARTs may vary among different tumors, but the overall impression is that CD4 CARTs may persist longer in vivo and generate durable antitumor effect. Two clinical trials showed that it is possible to generate sufficient CARTs from defined CD4 and CD8 T cells subsets (Turtle et al., 2016a; Turtle et al., 2016b). Further studies are needed to determine the antitumor efficacy of a defined combination of CD8 and CD4 CARTs in other tumor models.
Tcm and Tn: T cell subsets in peripheral blood are found in different differentiation stages, which include Tn (naïve), Tscm (stem-like memory), Tcm (central memory), and Tem (effector memory). They also possess different capacity of effector function, proliferation, and persistence after adoptive transfer (Berger et al., 2008; Gattinoni et al., 2011; Wang et al., 2011). CD19 CARTs generated from unfractionated T cells have achieved high response rates in treating B cell leukemia. As unfractionated T cells, regardless of their heterogeneity, should contain all subsets at different differentiation stages, it seems that no isolation of T cell subsets is needed. However, recent studies showed that memory T cells could drive the differentiation of naïve T cells and impaired their antitumor effects (Klebanoff et al., 2016; Xu and Dotti, 2016). Thus, purification of desired T cell subsets prior to CAR transduction and expansion may generate better CARTs. In addition, a precise composition of the T cell products and enrichment with the highest potential for engraftment and persistence may become particularly relevant in the setting of solid tumors (Hou et al., 2019). Using second generation CD19-41BB-CD3ζ and ROR1-41BB-CD3ζ CARs, Sommermeyer et al. studied whether CARTs generated from defined T cell subsets possessed different proliferation, survival capacity, and effector function (Sommermeyer et al., 2016). They found that Tn-derived CD4+CARTs and CD8+CARTs produced the highest levels of cytokines and that the CD4+CARTs generated from Tn and Tcm cells enhanced the antigen-driven proliferation of CD8+CARTs in culture. Remarkably, the combination of Tn- or Tcm-derived CD4+CARTs with Tcm-derived CD8+CARTs generated the strongest antitumor effects. Another study also showed that T cell subsets at different differentiation stages were equally transduced by CARs and that the Tn-derived CARTs exhibited the greatest rate of proliferation but lower cytokine production and killing activity (Schmueck-Henneresse et al., 2017). Clinical trial data also showed that adoptive transfer of a defined combination of CD4+CARTs and CD8+CARTs (as few as 1x105 cells/kg for CD4 and CD8) resulted in complete tumor remission in bone marrow in 93% of patients (Turtle et al., 2016a). However, because of the high complete response rate, it was impossible to compare the antitumor efficacy of bulk CD8 CARTs vs the Tcm-derived CD8 CARTs. In addition to CARTs from Tn and Tcm, a recent study showed that mesothelin-specific CARTs created from CD26hi (effector memory marker) CD4 T cells produced higher amounts of IL17 and could survive and persist longer in vivo, and possess potent antitumor effects (Bailey et al., 2017). Thus, the consensus view is that CARTs generated from defined (Tn or Tcm) CD4 and CD8 T cell subsets will generate strong antitumor effects, but the optimal T cell subsets for each particular CAR may require experimental data to determine. The role of the T cell subsets on generating effective TCRTs has not been carefully studied, but it is likely that the same principle may also be applied.
4.2.2. CAR design:
A CAR is a synthetic molecule consisting of an extracellular single-chain variable fragment of an antibody (scFv) (or a receptor ligand or even cytokines) that provides the CAR its specificity, a hinge that offers the flexibility of the scFv, a transmembrane (TM) domain that anchors the scFv to the T cells, and an intracellular signaling domain (ICD) that transmits the signal and activates the T cells (Zhao et al., 2015). Each of these components potentially affects the function of the CARTs (Guedan et al., 2019). We briefly summarized the essence of these components, and readers are encouraged to read details from two recent reviews (Guedan et al., 2019; Stoiber et al., 2019).
-
scFv: Most scFvs in CAR designs are derived from mouse or human antibodies, even though some studies do use nanobodies from Camilidae (De Munter et al., 2018).
Targets: Ideally, the scFv should target tumor-specific antigens. But, such tumor-specific antigens are rare if not impossible to find. Instead, overexpressed tumor-associated antigens are frequently used as targets. Due to the differential level of antigen between tumor cells and normal cells, a treatment window exists that can kill tumor cells without significant toxicity to normal cells. As aforementioned, another approach to reduce toxicity is by creating dual targeting CARs (Orentas et al., 2017).
Affinity: CARTs constructed from high-affinity scFv might mediate better antitumor reactivity (Hudecek et al., 2013), but may also kill normal cells expressing low levels of the target antigen. In addition, the scFvs of higher affinities may incur higher background tonic signaling (Lynn et al., 2019). Thus, the scFv affinity may be tuned to maximize the differential effects of CARTs against tumor targets from normal cells (Liu et al., 2015) and to minimize the tonic signaling.
Epitope location: The antibody binding sites on target antigen also play an important role in determining CART efficacy. scFvs targeting the membrane-proximal epitopes may generate more effective CARTs (Hombach et al., 2007; James et al., 2008), possibly because of better immunological synapse and stronger signaling (Li et al., 2017a; Xiong et al., 2018). Again, stronger signaling may accompany higher tonic signaling which may predispose CARTs to exhaustion.
Order of the variable region of the light chain (VL) and the variable region of the heavy chain (VH), and the choice of the linker: Both configurations, the VL-linker-VH and VH-linker-VL, have been used to create scFvs (Burns et al., 2010; Desplancq et al., 1994). The studies found that, depending on the CAR, the linker and the order of the VL and VH regions could affect the expression and the binding of the target antigen by the CAR. Although, Burns et al ( 2010) showed that the VL-linker-VH configuration bearing the linker sequence GSTSGSGKPGSEGS generated better CART function than the VH-linker-VL bearing a GS linker (GGGGSGGGGSGGGGS), most recent studies still use the GS linker (Rafiq et al., 2017; Richman et al., 2018).
Hinge: The hinge is a spacer that separates the scFv from the TM domain. It confers stability to the CAR and the flexibility to bind the target antigen on tumor cells. Currently, there are 2 classes of hinge being used to create CARs, the Fc-containing IgG1, G2, and G4 antibody-derived hinges, and the native CD28 and CD8 derived hinges. Most CARs utilized the CD8α and CD28 spacers. Longer Ig-derived hinges offer more flexibility, but their Fc may interact with FcR+ myeloid cells and result in activation induced T cell death (Hudecek et al., 2015), or possible tonic signaling (Frigault et al., 2015). Mutation of the IgG4 Fc spacer avoids FcR binding, and can improve T cell persistence and antitumor efficacy (Jonnalagadda et al., 2015). The choice of the length of the hinge may be affected by the location of the CAR-targeted epitope. To help CARs reach membrane-proximal epitopes, longer hinges may be required (Guest et al., 2005). In contrast, a short hinge may be more effective for binding membrane-distal epitopes (Hudecek et al., 2013; Hudecek et al., 2015). The length of the hinge may also affect the formation of the immunological synapse (Srivastava and Riddell, 2015). Thus, the choice of the hinge and its length need to be optimized for each CAR in order to generate effective CARTs.
TM: The TM domain is mainly taken from CD8α and CD28, possibly because it may increase CAR expression compared to the original CD3ζ TM (Zhang et al., 2012). Some recent studies also used ICOS TM (Guedan et al., 2018). The TM domain may be involved in bringing the CAR molecules together and thus helps in the formation of the immunological synapse (Bridgeman et al., 2010). The selection of the TM is normally associated to the membrane-proximal costimulatory molecules, which may favor CART activation (Guedan et al., 2018).
Intracellular domain (ICD): The ICD is a critical component of a CAR that properly transmits the signal and activates the CARTs. Most CAR ICDs include CD3ζ, 41BB, and/or CD28 signaling domains, even though other TNF receptor family (OX40 and CD27) and ICOS have also been used. Based on the ICD, there are 3 generations of CARs. The 1st generation of CARs contains the CD3ζ only, the 2nd generation contains CD3ζ combined with 4-1BB or CD28 endodomains, and the 3rd generation contains CD3ζ with a combination of two signaling domains. The consensual point of view on the ICD is that CD28 CARTs expand fast but persist for a short time, while the 4-1BB-containing CARTs expand slower, but persist longer (Zhao et al., 2015). It is not clear whether the same trend will be true for CARTs targeting solid tumors. In a comparative study, a 3rd generation ICOS-BB-z CAR targeting mesothelin showed enhanced persistence of CD4 and CD8 CART cells, better antitumor effects and T cell survival in solid tumor models compared to BBz CARTs (Guedan et al., 2018). In addition, adjustment of the number of immunoreceptor tyrosine-based activation motifs (ITAMs) in the ICD can modulate tonic signaling, CART activation and exhaustion, and thus change their fate and antitumor effect (Feucht et al., 2019).
4.2.3. Vector and promoter:
Both retroviral and lentiviral vectors are being used to deliver CAR or TCR genes into T cells to generate TCRTs and CARTs. But, the retroviral vector integration and the use of the long-terminal repeats (LTR) promoter may lead to silencing of the delivered genes (Ellis, 2005). Non-viral vectors, like piggyback (Morita et al., 2018) and sleepy beauty vector systems (Singh et al., 2013), are also being used for CAR gene transfer. Currently, lentiviral vectors are the most widely used vehicles for TCR and CAR gene transfer. Several promoters have been used to drive CAR and TCR gene expression. The retroviral LTR promoter drives higher CAR expression but also leads to T cell exhaustion due to tonic signaling (Gomes-Silva et al., 2017). Compared to CMV and PGK promoters, the EF1α promoter generated the highest CAR expression (Milone et al., 2009). While one study reported that high-CAR expressing populations expanded after antigen stimulation and possessed stronger function (Chang et al., 2015), most studies have showed that high CAR expression levels may be detrimental to CART function (Frigault et al., 2015; Guedan et al., 2018; Long et al., 2015). One study found that low CAR-expressing T cells driven by a short truncated PGK promoter persisted longer and generated better antitumor effects than the longer version of the promoter (Frigault et al., 2015). Inserting the CAR gene to the locus of endogenous T cell receptor (TRAC), and thus putting the CAR gene under the control of the TCR promoter (Eyquem et al., 2017), generated uniform CAR expression and enhanced CART potency.
4.2.4. CART production process:
The T cell production process also affects the quality of CARTs and TCRTs. The process includes in vitro activation of T cells, transduction, and expansion of the transduced cells. It is not clear whether the type of culture media affects CART survival and persistence. But, there are reports showing that addition of IL-7 and IL-15 increases the frequency of CD8+CD45RA+CCR7+ central memory T cells during the ex vivo expansion of CARTs (Xu et al., 2014). This suggests that the duration of the ex vivo culture and specific antigen expansion before adoptive transfer should be optimized to maximize the in vivo persistence and function of CARTs.
4.3. Strategies to create effective CARTs:
The aforementioned parameters (T cell subsets, CAR molecule design, CAR gene delivery vector, promoters, and the CART production process) can be manipulated and optimized to enhance the antitumor efficacy of CARTs. Additional factors may also be incorporated into the generation of CARTs to further improve their function. The ultimate goal is to create CARTs that can survive and persist longer, be able to home into tumor lesions, undergo antigen-driven expansion, resist immune suppression of the tumor milieu, and maintain better effector function without becoming exhausted. Even though the strategies have been designed for CARTs, some of them may be applicable to TCRTs.
4.3.1. Create CARTs with better survival and persistence:
Based on previous analysis, CD4 and CD8 subsets with Tn or Tcm phenotypes should be isolated and separately transduced to create CARTs. CARs with 4-1BB and ICOS costimulatory domains may help CART survival and persistence. The ICOS domain skewed CD4 T cells toward the Th17 subset and enhanced CD8 CART function by increasing their survival (Guedan et al., 2018). The promoter to drive CAR expression should be selected experimentally. The addition of IL7 and IL15 in the production media may also help generate younger CARTs that survive and persist better. A previous study showed that co-expression of a constitutively activated form of STAT5 (CA-STAT5) promoted long-live TCRTs and increased their antitumor effects after adoptive transfer in an animal model (Grange et al., 2012). It is likely that co-expression of CA-STAT5 may also help CARTs survive and persist longer. Several studies have tested the co-expression of cytokine and cytokine receptors, including the membrane-bound IL15/IL15Rα fusion protein (Hurton et al., 2016) and the IL4/IL21 receptor (Wang et al., 2019) in CARTs. Co-expression of the IL4/21 receptor enhanced IL17 production and increased CD26 expression, two of the hallmarks of long survival (Bailey et al., 2017; Bowers et al., 2017). One recent report showed that one single CART cell clone from a complete responder patient contained an insertional disruption in the TET2 gene. This CART clone had a significant long-life span, and may be responsible for the enhanced antitumor effect of the therapy (Fraietta et al., 2018). It would be interesting to study whether knocking-out the TET2 gene could enhance CART survival.
4.3.2. Enhance CART homing into tumor lesions:
One major difference between hematological cancer and solid tumors, such as HCC, is that CARTs need additional steps to migrate and infiltrate into tumor lesions to generate antitumor effects. One recent study found that co-expression of CCL19 and IL7 increased CART migration into solid tumor lesions (Adachi et al., 2018). Some conventional cancer therapies may significantly affect the microenvironment in tumor lesions to co-opt CART and TCRT therapy. For example, ionizing irradiation increases IL8 production in glioma cells. Huang and colleagues recently took advantage of the irradiation treatment- induced IL8 production in brain tumors and designed CD70 CARTs to co-express IL8 receptors, CXCR1 and CXCR2. Expression of these IL8 receptors on CARTs significantly enhanced CART infiltration and anti-tumor effects in brain tumors in mice (Jin et al., 2019).
4.3.3. Overcome T cell exhaustion:
T cell exhaustion is a major hurdle in CART and TCRT therapies, especially for solid tumors. T cell exhaustion is a state of T cell loss of effector function in a hierarchical manner. The first sign of T cell exhaustion is the loss of IL-2 production, then TNFα, followed by INFγ and β-chemokines (Wherry, 2011). The exhausted state is also accompanied by reduced proliferative abilities, upregulation of exhaustion markers such as PD-1, TIM3, LAG3, and CTLA-4, and altered metabolic fitness. T cell exhaustion is thought to be induced by excessive or chronic TCR stimulation with robust co-inhibitory signaling, or restricted co-stimulation (Hashimoto et al., 2018). Recent studies have demonstrated that exhausted CAR-T cells, similar to exhausted T cells, possess unique transcriptional and epigenetic signatures (Chen et al., 2019; Lynn et al., 2019; Seo et al., 2019). Philip et al. recently described two states of T cells dysfunction in mice. Only the T cells in the “plastic (CD101lowCD38lowCD5high)” dysfunctional state could be rescued (Philip et al., 2017), while cells in the “fixed (CD101highCD38highCD5low)”” state no longer reacted to cytokine supplementation or antigen stimulation. Here we briefly summarized approaches to reduce exhaustion and ways to rescue exhausted T cells.
Reduce tonic signaling: Antigen-independent tonic signaling is one of the factors that predisposes CARTs and TCRTs to exhaustion and short duration of survival. Many factors previously discussed may affect tonic signaling and thus T cell exhaustion (Martinez and Moon, 2019). Those factors include the density and affinity of CAR molecules on T cells and the choice of costimulatory domain (41BB generates less tonic signaling than CD28 ICD (Long et al., 2015)), the hinge, the gene expression vector, and even the order of the ICDs (41BB vs ICOS) in the 2nd and 3rd generation of CARs. Reducing the duration of the ex vivo culture may also help CART function (Ghassemi et al., 2018).
Target key factors: Several transcription factors have been identified as key players in driving T cell exhaustion (Saeidi et al., 2018). (A) Factors increasing exhaustion: Blimp-1 has been shown to induce T cell exhaustion in chronic parasite infection. Hwang et al showed that knockout of Blimp-1 could reverse exhaustion of CD4 and CD8 T cells (Hwang et al., 2016). Joyce Chen et al. demonstrated that NR4A transcription factors limit CAR-T function in solid tumors, and that triple knockout of the NR4A family in CAR-T Cells resulted in tumor regression and prolonged the survival of tumor-bearing mice (Chen et al., 2019). The triple knockout of NR4A family members in CAR-T cells exhibited a phenotype and gene expression profile commonly seen in CD8+ effector T cells (Chen et al., 2019). Later Khan et al. identified TOX as a central regulator in exhausted mouse CD8+T cells and showed that knockout of both TOX and TOX2 in CARTs generated more effective suppression of tumor growth and prolonged survival of tumor-bearing animals. The TOX transcription factors have been found to cooperate with the NR4A family to drive CD8+ T cell exhaustion. These studies suggest that reduction of TOX and NR4A expression will help generate more effective CARTs and TCRTs for cancer immunotherapy (Khan et al., 2019; Seo et al., 2019). Another factor affecting CART cell survival is the presence of their endogenous TCR. A study showed that TCR engagement negatively affects CD8 but not CD4 CAR T cell expansion and function (Yang et al., 2017), and insertion of the CAR gene into the TCR locus delayed CART exhaustion (Eyquem et al., 2017). (B) Factors resisting exhaustion: On the opposite, some transcription factors such as T-bet promote potent activity of CD4+ CARTs by skewing T cells towards the Th1 phenotype (Gacerez and Sentman, 2018). Co-expression of IL4/IL7 or IL4/IL21 receptors also made CARTs resistant to exhaustion (Wang et al., 2019). A latest study showed that co-expression of C-Jun overcame T cell exhaustion and enhanced antitumor effects (Lynn et al., 2019). (C): Moreover, CART function can be rescued by inhibition of apoptosis by Fas-Fc and DR5-Fc recombinant proteins (146), by disruption of PD-1 (147), by co-administration of checkpoint blockade (anti PD-1/PD-L1) (12), and by genetically introduction of dominant negative forms of PD-1 on CAR-T cells and/or co-expression of soluble PD1-IgG4 CH3 fusion proteins (148). In addition, inhibition of Lck activation in CARTs may confer Treg resistance due to disruption of IL2 production (Suryadevara et al., 2019).
4.3.4. Supercharge CARTs:
Engineering CARTs to co-express cytokines or other therapeutic factors to become “TRUCKs” (T cells redirected for universal cytokine-mediated killing) that make CARTs deliver the payload to tumor lesions has shown to enhance CART’s antitumor efficacy. A comprehensive review is recommended (Jaspers and Brentjens, 2017). Some of the payloads may enhance CART survival and persistence, and confer CARTs resistance to immune suppression and exhaustion, as mentioned previously. Other cytokines or cytokine receptors may affect the tumor microenvironment such as the IL4-IL7 chimeric receptor (Leen et al., 2014). Also, IL12 may have direct antitumor effects (Koneru et al., 2015a; Koneru et al., 2015b; Liu et al., 2019). Co-expression of a dominant negative TGF-β receptor (TGF-βRII) increased CART proliferation, cytokine secretion, resistance to exhaustion, and long-term in vivo persistence. This resulted in enhanced antitumor effects in an aggressive human prostate cancer mouse model (Kloss et al., 2018).
4.4. Safety of CARTs:
Several important safety concerns are related to CART therapies, such as cytokine release syndrome (CRS), central nervous system toxicity (CNS) and off-target or on-target/off-tumor recognition.
4.4.1. CRS:
In treating hematological cancers, the most common toxicity associated to CART therapy is the CRS. It has been reported that 92% of ALL patients treated with anti CD19 CARTs experienced CRS, and around 50% of these patients developed life threatening symptoms. Symptoms can range from flu-like symptoms like fever, fatigue, headache, etc. to more severe symptoms such as organ dysfunction (Yáñez, 2019). Some cytokines (IFNγ and TNFα), which are implicated in CRS are produced by the CARTs after interaction with tumor cells, and in some cases, with normal cells. These cytokines trigger the activation of other immune cells like macrophages, which in turn produce high levels of pro-inflammatory cytokines (IL-6, IL-1 and IL-10). For solid tumors, Hou, B. et al. performed a meta-analysis of 22 cases of clinical trials using CART therapy and did not find any report of serious CRS symptoms associated with the infusion of CARTs (87). We speculate that one possible reason for the high incidence of CRS associated with hematological cancers is the immediate engagement of large number of CARTs with target antigens in the blood resulting in fast cytokine release. In the solid tumor treatment, CARTs may migrate into tumor lesions in a progressive manner and thus may generate a mild CRS.
4.4.2. CNS:
Another common toxicity associated with CART infusion is the CART-related encephalopathy syndrome (CRES). In 90% of patients, CNS toxicity occurs along with CRS or after resolution of CRS. Even though the pathogenesis of CNS toxicity remains unclear, some studies indicate that the impairment of the blood brain barrier function by cytokines such as TNFα, IL-6, IL-1 along with the balance of angiotensin 1 and angiotensin 2 might be major leading factors (Yáñez, 2019). Symptoms in CNS toxicity vary from headache, dizziness, memory loss or tremor, to delirium and even coma. The management of CNS toxicity is based on the use of corticosteroids, given at different doses, and depending on the severity of the symptoms.
4.4.3. On-target/off-tumor and off-target toxicity:
Severe adverse effects can be also generated by the on-target/off-tumor recognition of the CARTs, especially when there is low-level expression of the target antigen in tissues other than tumors. Morgan et al. reported a case in which a patient experienced respiratory distress within 15 minutes after infusion of anti-ERBB2 CARTs, and died five days after treatment despite medical intervention (Morgan et al., 2010). Serum samples showed increased levels of IFN-γ, IL-6 and IL-10, granulocyte macrophage-colony stimulating factor (GM-CSF) and TNFα, thus indicating the presence of a cytokine storm. The respiratory distress is thought to be caused by low levels of ERBB2 in the lungs. Due to the promiscuity of TCRs, TCRTs may cross bind the human antigens other than the intended tumor target, thus TCRs could also generate severe off-target toxicity (please also see section 3.3).
4.4.4. Other toxicities:
Mercela et al. reported a case of a subject who developed anaphylaxis and cardiac arrest after treatment with anti-Mesothelin CARTs. That is the first case reported in which mRNA-CARTs induced a host immunologic response against CARs. It is believed that the adverse events were caused by the development of IgE antibodies as a result of intermittent administration of the mRNA-CARTs containing murine-derived parts. Other toxicities, such as tumor lysis syndrome, cytopenias, cardiac toxicity, hypogammaglobullinemia and graft-versus-host-disease are less common, but still preoccupying. Some of these toxicities are associated to the preparation and administration of the infusion product, while others depend on the patient’s conditioning previous to receiving the CART treatment. As already mentioned, a rare adverse event of disruption of a gene function, which resulted in patient’s complete remission and CART survival several years after the treatment with anti-CD19 CAR-T Cells was reported by Frajeta et al in 2018 (Fraietta et al., 2018). This patient had a single functional copy of the TET2 gene, which was disrupted by the lentiviral insertion of the CAR gene, resulting in a beneficial outcome. Normally lentiviral and retroviral vectors are considered safe for the delivery of DNA into somatic cells because they have been deprived of their replicative capacity. However, due to the random integration of both vector systems, there are rare chances of potential integrations leading to oncogenesis.
4.4.5. Strategies to overcome CART toxicity:
Along with the search of more specific tumor antigens, researchers have been developing strategies to minimize toxicities associated to CART immunotherapy.
Controlled removal of CARTs: Two suicide gene systems are being tested in human settings. A) The drug-inducible Caspase 9 system that leads to the rapid induction of apoptosis of the CARTs upon administration of a dimerizing drug (AP1903 or AP20187) (Gargett and Brown, 2014). B) A truncated epidermal growth factor receptor (EGFR) lacking intracellular domain. The truncated EGFR allows for the elimination of the CARTs using an antibody specific for the receptor (Paszkiewicz et al., 2016). This system requires the functionality of the patient’s lymphocytes in order to be effective. Thus, it might not be recommended in patients who undergo lymphodepletion before CART infusion.
Dual-CARTs: As aforementioned, Chen et al. developed dual-targeted CART cells (targeting GPC3 and ASGR1 CARs) (Chen et al., 2017). The dual-targeted CARTs produced higher levels of cytokines and had a greater anti-tumor effect than single-targeted CARTs. The dual-CARTs did not show anti-tumor effect against ASGR1+GPC3− cells, and showed potent anti-tumor effect against ASGR1+GPC3+ or ASGR1−GPC3+ cells. Only the dual-targeted, but not the single-targeted, CART cells were able to significantly inhibit tumor growth in MHCC-97L (GPC3+ASCR1+) and Huh-7 xenografts. The clinical safety of dual CARs remains to be evaluated.
Logic gate design: Another strategy includes splitting CAR signaling components to generate the output of an AND logic gate. The TM and extracellular domains of the CAR are physically separated from the costimulatory domains. Each fragment of the CAR contains a dimerizing moiety that allows them to re-assemble when a small molecule is added (Wu et al., 2015). Furthermore, it has been suggested to control CART function through the use of a split, universal, and programmable (SUPRA) CAR system that allows for tuning of T Cell activation, signal strength, or blocking the SUPRA CAR-T cells in case of adverse events (Cho et al., 2018).
Activation inhibitors: More recently, it was shown that a tyrosine kinase inhibitor, desatinib, can turn CARTs off by interfering with LCK signaling, leading to the transient inhibition of phosphorylation of ZAP70. Desatinib acts on both CD4+ and CD8+ CARTs by halting their cytolytic activity, cytokine production and proliferation for several days without affecting cell viability. The dose of desatinib can be titrated depending on desired levels of CAR-T inhibition. In a mouse model of CRS, the authors showed that desatinib was able to protect a proportion of mice from lethal CRS after CART infusion. It also showed that the effects of desatinib could be reversed after discontinuation of the drug. Moreover, desatinib possesses a great potential to be used as an emergency drug to rapidly block CAR-T cell activity in case of life-threatening events (Mestermann et al., 2019).
5. Concluding remarks
By far, the most successful HCC therapy is the PD-1/PD-L1 checkpoint blockade. However, it yields only 15-20% of overall response rate (Hato et al., 2014). To improve HCC immunotherapy, cell therapies, especially the engineered TCRTs and CARTs are actively being studied. Interrogating each component of the CAR and TCR design and selecting the optimal T cell subsets may help create more effective and safe TCRTs and CARTs. The combination of engineered T cells with cancer vaccines and checkpoint blockade may greatly improve the therapeutic outcome of HCC immunotherapy.
Fig. 3.
Parameters may affect the antitumor efficacy of CARTs. 1. T cell subsets to be engineered; 2. The vector and promoter to carry and express CARs; 3. The CAR design; 4. The production process of CARTs. LTR, long-terminal repeats of retroviral vectors; TM, transmembrane domain; Tcm, central memory T cells; Tn, Naïve T cells; VL, variable light chain of antibody; VH, variable heavy chain of antibody; ICD, Intracellular domain.
6. Acknowledgement
Research in Dr. Yukai He’s laboratory on developing TCRTs and CART for HCC immunotherapy has been supported by NHI/NCI grants (R01CA168912 and R01CA235159) and Augusta University intramural grant. We would like to thank Ms. Yibing Peng and all previous members of Dr. Yukai He’s laboratory for their contribution on developing the HCC specific TCRT and CART projects.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abou-Alfa GK, Puig O, Daniele B, Kudo M, Merle P, Park JW, Ross P, Peron JM, Ebert O, Chan S, Poon TP, Colombo M, Okusaka T, Ryoo BY, Minguez B, Tanaka T, Ohtomo T, Ukrainskyj S, Boisserie F, Rutman O, Chen YC, Xu C, Shochat E, Jukofsky L, Reis B, Chen G, Di Laurenzio L, Lee R, Yen CJ, 2016. Randomized phase II placebo controlled study of codrituzumab in previously treated patients with advanced hepatocellular carcinoma. J Hepatol 65, 289–295. [DOI] [PubMed] [Google Scholar]
- Adachi K, Kano Y, Nagai T, Okuyama N, Sakoda Y, Tamada K, 2018. IL-7 and CCL19 expression in CAR-T cells improves immune cell infiltration and CAR-T cell survival in the tumor. Nat Biotechnol 36, 346–351. [DOI] [PubMed] [Google Scholar]
- Alizadeh D, Wong RA, Yang X, Wang D, Pecoraro JR, Kuo CF, Aguilar B, Qi Y, Ann DK, Starr R, Urak R, Wang X, Forman SJ, Brown CE, 2019. IL15 enhances CAR-T cell antitumor activity by reducing mTORC1 activity and preserving their stem cell memory phenotype. Cancer Immunol Res 7, 759–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey SR, Nelson MH, Majchrzak K, Bowers JS, Wyatt MM, Smith AS, Neal LR, Shirai K, Carpenito C, June CH, Zilliox MJ, Paulos CM, 2017. Human CD26(high) T cells elicit tumor immunity against multiple malignancies via enhanced migration and persistence. Nat Commun 8, 1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger C, Jensen MC, Lansdorp PM, Gough M, Elliott C, Riddell SR, 2008. Adoptive transfer of effector CD8+ T cells derived from central memory cells establishes persistent T cell memory in primates. J Clin Invest 118, 294–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Border EC, Sanderson JP, Weissensteiner T, Gerry AB, Pumphrey NJ, 2019. Affinity-enhanced T-cell receptors for adoptive T-cell therapy targeting MAGE-A10: strategy for selection of an optimal candidate. Oncoimmunology 8, e1532759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers JS, Nelson MH, Majchrzak K, Bailey SR, Rohrer B, Kaiser AD, Atkinson C, Gattinoni L, Paulos CM, 2017. Th17 cells are refractory to senescence and retain robust antitumor activity after long-term ex vivo expansion. JCI Insight 2, e90772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brar G, Greten TF, Brown ZJ, 2018. Current frontline approaches in the management of hepatocellular carcinoma: the evolving role of immunotherapy. Therap Adv Gastroenterol 11, 1756284818808086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brichard VG, Louahed J, Clay TM, 2013. Cancer regression and neurological toxicity cases after anti-MAGE-A3 TCR gene therapy. J Immunother 36, 79–81. [DOI] [PubMed] [Google Scholar]
- Bridgeman JS, Hawkins RE, Bagley S, Blaylock M, Holland M, Gilham DE, 2010. The optimal antigen response of chimeric antigen receptors harboring the CD3zeta transmembrane domain is dependent upon incorporation of the receptor into the endogenous TCR/CD3 complex. J Immunol 184, 6938–6949. [DOI] [PubMed] [Google Scholar]
- Buonaguro L, Mauriello A, Cavalluzzo B, Petrizzo A, Tagliamonte M, 2019. Immunotherapy in hepatocellular carcinoma. Ann Hepatol 18, 291–297. [DOI] [PubMed] [Google Scholar]
- Burns WR, Zhao Y, Frankel TL, Hinrichs CS, Zheng Z, Xu H, Feldman SA, Ferrone S, Rosenberg SA, Morgan RA, 2010. A high molecular weight melanoma-associated antigen-specific chimeric antigen receptor redirects lymphocytes to target human melanomas. Cancer Res 70, 3027–3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterfield LH, Koh A, Meng W, Vollmer CM, Ribas A, Dissette V, Lee E, Glaspy JA, McBride WH, Economou JS, 1999. Generation of human T-cell responses to an HLA-A2.1-restricted peptide epitope derived from alpha-fetoprotein. Cancer Res 59, 3134–3142. [PubMed] [Google Scholar]
- Butterfield LH, Meng WS, Koh A, Vollmer CM, Ribas A, Dissette VB, Faull K, Glaspy JA, McBride WH, Economou JS, 2001. T cell responses to HLA-A*0201-restricted peptides derived from human alpha fetoprotein. J Immunol 166, 5300–5308. [DOI] [PubMed] [Google Scholar]
- Butterfield LH, Ribas A, Dissette VB, Lee Y, Yang JQ, De la Rocha P, Duran SD, Hernandez J, Seja E, Potter DM, McBride WH, Finn R, Glaspy JA, Economou JS, 2006. A phase I/II trial testing immunization of hepatocellular carcinoma patients with dendritic cells pulsed with four alpha-fetoprotein peptides. Clin Cancer Res 12, 2817–2825. [DOI] [PubMed] [Google Scholar]
- Caballero OL, Chen YT, 2009. Cancer/testis (CT) antigens: potential targets for immunotherapy. Cancer Sci 100, 2014–2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron BJ, Gerry AB, Dukes J, Harper JV, Kannan V, Bianchi FC, Grand F, Brewer JE, Gupta M, Plesa G, Bossi G, Vuidepot A, Powlesland AS, Legg A, Adams KJ, Bennett AD, Pumphrey NJ, Williams DD, Binder-Scholl G, Kulikovskaya I, Levine BL, Riley JL, Varela-Rohena A, Stadtmauer EA, Rapoport AP, Linette GP, June CH, Hassan NJ, Kalos M, Jakobsen BK, 2013. Identification of a Titin-derived HLA-A1-presented peptide as a cross-reactive target for engineered MAGE A3-directed T cells. Sci Transl Med 5, 197ra103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang MH, You SL, Chen CJ, Liu CJ, Lee CM, Lin SM, Chu HC, Wu TC, Yang SS, Kuo HS, Chen DS, Taiwan Hepatoma Study G, 2009. Decreased incidence of hepatocellular carcinoma in hepatitis B vaccinees: a 20-year follow-up study. J Natl Cancer Inst 101, 1348–1355. [DOI] [PubMed] [Google Scholar]
- Chang ZL, Silver PA, Chen YY, 2015. Identification and selective expansion of functionally superior T cells expressing chimeric antigen receptors. J Transl Med 13, 161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Li K, Jiang H, Song F, Gao H, Pan X, Shi B, Bi Y, Wang H, Wang H, Li Z, 2017. Development of T cells carrying two complementary chimeric antigen receptors against glypican-3 and asialoglycoprotein receptor 1 for the treatment of hepatocellular carcinoma. Cancer Immunol Immunother 66, 475–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Lopez-Moyado IF, Seo H, Lio CJ, Hempleman LJ, Sekiya T, Yoshimura A, Scott-Browne JP, Rao A, 2019. NR4A transcription factors limit CAR T cell function in solid tumours. Nature 567, 530–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho JH, Collins JJ, Wong WW, 2018. Universal chimeric antigen receptors for multiplexed and logical control of T cell responses. Cell 173, 1426–1438 e1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Angelo SP, Melchiori L, Merchant MS, Bernstein D, Glod J, Kaplan R, Grupp S, Tap WD, Chagin K, Binder GK, Basu S, Lowther DE, Wang R, Bath N, Tipping A, Betts G, Ramachandran I, Navenot JM, Zhang H, Wells DK, Van Winkle E, Kari G, Trivedi T, Holdich T, Pandite L, Amado R, Mackall CL, 2018. Antitumor activity associated with prolonged persistence of adoptively transferred NY-ESO-1 (c259)T cells in synovial sarcoma. Cancer Discov 8, 944–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dargel C, Bassani-Sternberg M, Hasreiter J, Zani F, Bockmann JH, Thiele F, Bohne F, Wisskirchen K, Wilde S, Sprinzl MF, Schendel DJ, Krackhardt AM, Uckert W, Wohlleber D, Schiemann M, Stemmer K, Heikenwalder M, Busch DH, Richter G, Mann M, Protzer U, 2015. T cells engineered to express a T-cell receptor specific for glypican-3 to recognize and kill hepatoma cells in vitro and in mice. Gastroenterology 149, 1042–1052. [DOI] [PubMed] [Google Scholar]
- De Munter S, Ingels J, Goetgeluk G, Bonte S, Pille M, Weening K, Kerre T, Abken H, Vandekerckhove B, 2018. Nanobody Based Dual Specific CARs. Int J Mol Sci 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desplancq D, King DJ, Lawson AD, Mountain A, 1994. Multimerization behaviour of single chain Fv variants for the tumour-binding antibody B72.3. Protein Eng 7, 1027–1033. [DOI] [PubMed] [Google Scholar]
- Docta RY, Ferronha T, Sanderson JP, Weissensteiner T, Pope GR, Bennett AD, Pumphrey NJ, Ferjentsik Z, Quinn LL, Wiedermann GE, Anderson VE, Saini M, Maroto M, Norry E, Gerry AB, 2019. Tuning T-cell receptor affinity to optimize clinical risk-benefit when targeting alpha-fetoprotein-positive liver cancer. Hepatology 69, 2061–2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Serag HB, Tran T, Everhart JE, 2004. Diabetes increases the risk of chronic liver disease and hepatocellular carcinoma. Gastroenterology 126, 460–468. [DOI] [PubMed] [Google Scholar]
- Ellis J, 2005. Silencing and variegation of gammaretrovirus and lentivirus vectors. Hum Gene Ther 16, 1241–1246. [DOI] [PubMed] [Google Scholar]
- Eyquem J, Mansilla-Soto J, Giavridis T, van der Stegen SJ, Hamieh M, Cunanan KM, Odak A, Gonen M, Sadelain M, 2017. Targeting a CAR to the TRAC locus with CRISPR/Cas9 enhances tumour rejection. Nature 543, 113–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng M, Ho M, 2014. Glypican-3 antibodies: a new therapeutic target for liver cancer. FEBS Lett 588, 377–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feucht J, Sun J, Eyquem J, Ho YJ, Zhao Z, Leibold J, Dobrin A, Cabriolu A, Hamieh M, Sadelain M, 2019. Calibration of CAR activation potential directs alternative T cell fates and therapeutic potency. Nat Med 25, 82–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filmus J, Capurro M, 2013. Glypican-3: a marker and a therapeutic target in hepatocellular carcinoma. FEBS J 280, 2471–2476. [DOI] [PubMed] [Google Scholar]
- Fraietta JA, Nobles CL, Sammons MA, Lundh S, Carty SA, Reich TJ, Cogdill AP, Morrissette JJD, DeNizio JE, Reddy S, Hwang Y, Gohil M, Kulikovskaya I, Nazimuddin F, Gupta M, Chen F, Everett JK, Alexander KA, Lin-Shiao E, Gee MH, Liu X, Young RM, Ambrose D, Wang Y, Xu J, Jordan MS, Marcucci KT, Levine BL, Garcia KC, Zhao Y, Kalos M, Porter DL, Kohli RM, Lacey SF, Berger SL, Bushman FD, June CH, Melenhorst JJ, 2018. Disruption of TET2 promotes the therapeutic efficacy of CD19-targeted T cells. Nature 558, 307–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frigault MJ, Lee J, Basil MC, Carpenito C, Motohashi S, Scholler J, Kawalekar OU, Guedan S, McGettigan SE, Posey AD Jr., Ang S, Cooper LJ, Platt JM, Johnson FB, Paulos CM, Zhao Y, Kalos M, Milone MC, June CH, 2015. Identification of chimeric antigen receptors that mediate constitutive or inducible proliferation of T cells. Cancer Immunol Res 3, 356–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gacerez AT, Sentman CL, 2018. T-bet promotes potent antitumor activity of CD4+CAR T cells. Cancer Gene Ther 25, 117–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao H, Li K, Tu H, Pan X, Jiang H, Shi B, Kong J, Wang H, Yang S, Gu J, Li Z, 2014. Development of T cells redirected to glypican-3 for the treatment of hepatocellular carcinoma. Clin Cancer Res 20, 6418–6428. [DOI] [PubMed] [Google Scholar]
- Gao Q, Wang XY, Qiu SJ, Yamato I, Sho M, Nakajima Y, Zhou J, Li BZ, Shi YH, Xiao YS, Xu Y, Fan J, 2009. Overexpression of PD-L1 significantly associates with tumor aggressiveness and postoperative recurrence in human hepatocellular carcinoma. Clin Cancer Res 15, 971–979. [DOI] [PubMed] [Google Scholar]
- Gargett T, Brown MP, 2014. The inducible caspase-9 suicide gene system as a "safety switch" to limit on-target, off-tumor toxicities of chimeric antigen receptor T cells. Front Pharmacol 5, 235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattinoni L, Lugli E, Ji Y, Pos Z, Paulos CM, Quigley MF, Almeida JR, Gostick E, Yu Z, Carpenito C, Wang E, Douek DC, Price DA, June CH, Marincola FM, Roederer M, Restifo NP, 2011. A human memory T cell subset with stem cell-like properties. Nat Med 17, 1290–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring AJ, Xue SA, Ho ZZ, Teoh D, Ruedl C, Chia A, Koh S, Lim SG, Maini MK, Stauss H, Bertoletti A, 2011. Engineering virus-specific T cells that target HBV infected hepatocytes and hepatocellular carcinoma cell lines. J Hepatol 55, 103–110. [DOI] [PubMed] [Google Scholar]
- Ghassemi S, Nunez-Cruz S, O'Connor RS, Fraietta JA, Patel PR, Scholler J, Barrett DM, Lundh SM, Davis MM, Bedoya F, Zhang C, Leferovich J, Lacey SF, Levine BL, Grupp SA, June CH, Melenhorst JJ, Milone MC, 2018. Reducing ex vivo culture improves the antileukemic activity of chimeric antigen receptor (CAR) T cells. Cancer Immunol Res 6, 1100–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Global Burden of Disease Cancer, C., Fitzmaurice C, Allen C, Barber RM, Barregard L, Bhutta ZA, Brenner H, Dicker DJ, Chimed-Orchir O, Dandona R, Dandona L, Fleming T, Forouzanfar MH, Hancock J, Hay RJ, Hunter-Merrill R, Huynh C, Hosgood HD, Johnson CO, Jonas JB, Khubchandani J, Kumar GA, Kutz M, Lan Q, Larson HJ, Liang X, Lim SS, Lopez AD, MacIntyre MF, Marczak L, Marquez N, Mokdad AH, Pinho C, Pourmalek F, Salomon JA, Sanabria JR, Sandar L, Sartorius B, Schwartz SM, Shackelford KA, Shibuya K, Stanaway J, Steiner C, Sun J, Takahashi K, Vollset SE, Vos T, Wagner JA, Wang H, Westerman R, Zeeb H, Zoeckler L, Abd-Allah F, Ahmed MB, Alabed S, Alam NK, Aldhahri SF, Alem G, Alemayohu MA, Ali R, Al-Raddadi R, Amare A, Amoako Y, Artaman A, Asayesh H, Atnafu N, Awasthi A, Saleem HB, Barac A, Bedi N, Bensenor I, Berhane A, Bernabe E, Betsu B, Binagwaho A, Boneya D, Campos-Nonato I, Castaneda-Orjuela C, Catala-Lopez F, Chiang P, Chibueze C, Chitheer A, Choi JY, Cowie B, Damtew S, das Neves J, Dey S, Dharmaratne S, Dhillon P, Ding E, Driscoll T, Ekwueme D, Endries AY, Farvid M, Farzadfar F, Fernandes J, Fischer F, TT GH, Gebru A, Gopalani S, Hailu A, Horino M, Horita N, Husseini A, Huybrechts I, Inoue M, Islami F, Jakovljevic M, James S, Javanbakht M, Jee SH, Kasaeian A, Kedir MS, Khader YS, Khang YH, Kim D, Leigh J, Linn S, Lunevicius R, El Razek HMA, Malekzadeh R, Malta DC, Marcenes W, Markos D, Melaku YA, Meles KG, Mendoza W, Mengiste DT, Meretoja TJ, Miller TR, Mohammad KA, Mohammadi A, Mohammed S, Moradi-Lakeh M, Nagel G, Nand D, Le Nguyen Q, Nolte S, Ogbo FA, Oladimeji KE, Oren E, Pa M, Park EK, Pereira DM, Plass D, Qorbani M, Radfar A, Rafay A, Rahman M, Rana SM, Soreide K, Satpathy M, Sawhney M, Sepanlou SG, Shaikh MA, She J, Shiue I, Shore HR, Shrime MG, So S, Soneji S, Stathopoulou V, Stroumpoulis K, Sufiyan MB, Sykes BL, Tabares-Seisdedos R, Tadese F, Tedla BA, Tessema GA, Thakur JS, Tran BX, Ukwaja KN, Uzochukwu BSC, Vlassov VV, Weiderpass E, Wubshet Terefe M, Yebyo HG, Yimam HH, Yonemoto N, Younis MZ, Yu C, Zaidi Z, Zaki MES, Zenebe ZM, Murray CJL, Naghavi M, 2017. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-years for 32 Cancer Groups, 1990 to 2015: A systematic analysis for the global burden of disease study. JAMA Oncol 3, 524–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnjatic S, Nishikawa H, Jungbluth AA, Gure AO, Ritter G, Jager E, Knuth A, Chen YT, Old LJ, 2006. NY-ESO-1: review of an immunogenic tumor antigen. Adv Cancer Res 95, 1–30. [DOI] [PubMed] [Google Scholar]
- Gomes-Silva D, Mukherjee M, Srinivasan M, Krenciute G, Dakhova O, Zheng Y, Cabral JMS, Rooney CM, Orange JS, Brenner MK, Mamonkin M, 2017. Tonic 4-1BB costimulation in chimeric antigen receptors impedes T cell survival and is vector-dependent. Cell Rep 21, 17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grange M, Buferne M, Verdeil G, Leserman L, Schmitt-Verhulst AM, Auphan-Anezin N, 2012. Activated STAT5 promotes long-lived cytotoxic CD8+ T cells that induce regression of autochthonous melanoma. Cancer Res 72, 76–87. [DOI] [PubMed] [Google Scholar]
- Guedan S, Calderon H, Posey AD Jr., Maus MV, 2019. Engineering and design of chimeric antigen receptors. Mol Ther Methods Clin Dev 12, 145–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guedan S, Posey AD Jr., Shaw C, Wing A, Da T, Patel PR, McGettigan SE, Casado-Medrano V, Kawalekar OU, Uribe-Herranz M, Song D, Melenhorst JJ, Lacey SF, Scholler J, Keith B, Young RM, June CH, 2018. Enhancing CAR T cell persistence through ICOS and 4-1BB costimulation. JCI Insight 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guest RD, Hawkins RE, Kirillova N, Cheadle EJ, Arnold J, O'Neill A, Irlam J, Chester KA, Kemshead JT, Shaw DM, Embleton MJ, Stern PL, Gilham DE, 2005. The role of extracellular spacer regions in the optimal design of chimeric immune receptors: evaluation of four different scFvs and antigens. J Immunother 28, 203–211. [DOI] [PubMed] [Google Scholar]
- Hashimoto M, Kamphorst AO, Im SJ, Kissick HT, Pillai RN, Ramalingam SS, Araki K, Ahmed R, 2018. CD8 T Cell Exhaustion in Chronic Infection and Cancer: Opportunities for Interventions. Annu Rev Med 69, 301–318. [DOI] [PubMed] [Google Scholar]
- Hato T, Goyal L, Greten TF, Duda DG, Zhu AX, 2014. Immune checkpoint blockade in hepatocellular carcinoma: current progress and future directions. Hepatology 60, 1776–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Zhang J, Mi Z, Robbins P, Falo LD Jr., 2005. Immunization with lentiviral vector-transduced dendritic cells induces strong and long-lasting T cell responses and therapeutic immunity. J Immunol 174, 3808–3817. [DOI] [PubMed] [Google Scholar]
- Hombach AA, Schildgen V, Heuser C, Finnern R, Gilham DE, Abken H, 2007. T cell activation by antibody-like immunoreceptors: the position of the binding epitope within the target molecule determines the efficiency of activation of redirected T cells. J Immunol 178, 4650–4657. [DOI] [PubMed] [Google Scholar]
- Hong Y, Peng Y, Guo ZS, Guevara-Patino J, Pang J, Butterfield LH, Mivechi NF, Munn DH, Bartlett DL, He Y, 2014. Epitope-optimized alpha-fetoprotein genetic vaccines prevent carcinogen-induced murine autochthonous hepatocellular carcinoma. Hepatology 59, 1448–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou B, Tang Y, Li W, Zeng Q, Chang D, 2019. Efficiency of CAR-T therapy for treatment of solid tumor in clinical trials: a meta-analysis. Dis Markers 2019, 3425291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudecek M, Lupo-Stanghellini MT, Kosasih PL, Sommermeyer D, Jensen MC, Rader C, Riddell SR, 2013. Receptor affinity and extracellular domain modifications affect tumor recognition by ROR1-specific chimeric antigen receptor T cells. Clin Cancer Res 19, 3153–3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudecek M, Sommermeyer D, Kosasih PL, Silva-Benedict A, Liu L, Rader C, Jensen MC, Riddell SR, 2015. The nonsignaling extracellular spacer domain of chimeric antigen receptors is decisive for in vivo antitumor activity. Cancer Immunol Res 3, 125–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurton LV, Singh H, Najjar AM, Switzer KC, Mi T, Maiti S, Olivares S, Rabinovich B, Huls H, Forget MA, Datar V, Kebriaei P, Lee DA, Champlin RE, Cooper LJ, 2016. Tethered IL-15 augments antitumor activity and promotes a stem-cell memory subset in tumor-specific T cells. Proc Natl Acad Sci U S A 113, E7788–E7797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang S, Cobb DA, Bhadra R, Youngblood B, Khan IA, 2016. Blimp-1-mediated CD4 T cell exhaustion causes CD8 T cell dysfunction during chronic toxoplasmosis. J Exp Med 213, 1799–1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James SE, Greenberg PD, Jensen MC, Lin Y, Wang J, Till BG, Raubitschek AA, Forman SJ, Press OW, 2008. Antigen sensitivity of CD22-specific chimeric TCR is modulated by target epitope distance from the cell membrane. J Immunol 180, 7028–7038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaspers JE, Brentjens RJ, 2017. Development of CAR T cells designed to improve antitumor efficacy and safety. Pharmacol Ther 178, 83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen MC, Riddell SR, 2014. Design and implementation of adoptive therapy with chimeric antigen receptor-modified T cells. Immunol Rev 257, 127–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Z, Jiang X, Chen S, Lai Y, Wei X, Li B, Lin S, Wang S, Wu Q, Liang Q, Liu Q, Peng M, Yu F, Weng J, Du X, Pei D, Liu P, Yao Y, Xue P, Li P, 2016. Anti-GPC3-CAR T Cells Suppress the Growth of Tumor Cells in Patient-Derived Xenografts of Hepatocellular Carcinoma. Front Immunol 7, 690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin L, Tao H, Karachi A, Long Y, Hou AY, Na M, Dyson KA, Grippin AJ, Deleyrolle LP, Zhang W, Rajon DA, Wang QJ, Yang JC, Kresak JL, Sayour EJ, Rahman M, Bova FJ, Lin Z, Mitchell DA, Huang J, 2019. CXCR1- or CXCR2-modified CAR T cells co-opt IL-8 for maximal antitumor efficacy in solid tumors. Nat Commun 10, 4016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonnalagadda M, Mardiros A, Urak R, Wang X, Hoffman LJ, Bernanke A, Chang WC, Bretzlaff W, Starr R, Priceman S, Ostberg JR, Forman SJ, Brown CE, 2015. Chimeric antigen receptors with mutated IgG4 Fc spacer avoid fc receptor binding and improve T cell persistence and antitumor efficacy. Mol Ther 23, 757–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao WY, Su CW, Chau GY, Lui WY, Wu CW, Wu JC, 2011. A comparison of prognosis between patients with hepatitis B and C virus-related hepatocellular carcinoma undergoing resection surgery. World J Surg 35, 858–867. [DOI] [PubMed] [Google Scholar]
- Khan O, Giles JR, McDonald S, Manne S, Ngiow SF, Patel KP, Werner MT, Huang AC, Alexander KA, Wu JE, Attanasio J, Yan P, George SM, Bengsch B, Staupe RP, Donahue G, Xu W, Amaravadi RK, Xu X, Karakousis GC, Mitchell TC, Schuchter LM, Kaye J, Berger SL, Wherry EJ, 2019. TOX transcriptionally and epigenetically programs CD8(+) T cell exhaustion. Nature 571, 211–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebanoff CA, Scott CD, Leonardi AJ, Yamamoto TN, Cruz AC, Ouyang C, Ramaswamy M, Roychoudhuri R, Ji Y, Eil RL, Sukumar M, Crompton JG, Palmer DC, Borman ZA, Clever D, Thomas SK, Patel S, Yu Z, Muranski P, Liu H, Wang E, Marincola FM, Gros A, Gattinoni L, Rosenberg SA, Siegel RM, Restifo NP, 2016. Memory T cell-driven differentiation of naive cells impairs adoptive immunotherapy. J Clin Invest 126, 318–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloss CC, Lee J, Zhang A, Chen F, Melenhorst JJ, Lacey SF, Maus MV, Fraietta JA, Zhao Y, June CH, 2018. Dominant-negative TGF-beta receptor enhances PSMA-targeted human CAR T cell proliferation and augments prostate cancer eradication. Mol Ther 26, 1855–1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knolle PA, Thimme R, 2014. Hepatic immune regulation and its involvement in viral hepatitis infection. Gastroenterology 146, 1193–1207. [DOI] [PubMed] [Google Scholar]
- Kobayashi E, Mizukoshi E, Kishi H, Ozawa T, Hamana H, Nagai T, Nakagawa H, Jin A, Kaneko S, Muraguchi A, 2013. A new cloning and expression system yields and validates TCRs from blood lymphocytes of patients with cancer within 10 days. Nat Med 19, 1542–1546. [DOI] [PubMed] [Google Scholar]
- Komori H, Nakatsura T, Senju S, Yoshitake Y, Motomura Y, Ikuta Y, Fukuma D, Yokomine K, Harao M, Beppu T, Matsui M, Torigoe T, Sato N, Baba H, Nishimura Y, 2006. Identification of HLA-A2- or HLA-A24-restricted CTL epitopes possibly useful for glypican-3-specific immunotherapy of hepatocellular carcinoma. Clin Cancer Res 12, 2689–2697. [DOI] [PubMed] [Google Scholar]
- Koneru M, O'Cearbhaill R, Pendharkar S, Spriggs DR, Brentjens RJ, 2015a. A phase I clinical trial of adoptive T cell therapy using IL-12 secreting MUC-16(ecto) directed chimeric antigen receptors for recurrent ovarian cancer. J Transl Med 13, 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koneru M, Purdon TJ, Spriggs D, Koneru S, Brentjens RJ, 2015b. IL-12 secreting tumor-targeted chimeric antigen receptor T cells eradicate ovarian tumors in vivo. Oncoimmunology 4, e994446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korangy F, Ormandy LA, Bleck JS, Klempnauer J, Wilkens L, Manns MP, Greten TF, 2004. Spontaneous tumor-specific humoral and cellular immune responses to NY-ESO-1 in hepatocellular carcinoma. Clin Cancer Res 10, 4332–4341. [DOI] [PubMed] [Google Scholar]
- Krenciute G, Prinzing BL, Yi Z, Wu MF, Liu H, Dotti G, Balyasnikova IV, Gottschalk S, 2017. Transgenic expression of IL15 improves antiglioma activity of IL13Ralpha2-CAR T cells but results in antigen loss variants. Cancer Immunol Res 5, 571–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence MS, Stojanov P, Polak P, Kryukov GV, Cibulskis K, Sivachenko A, Carter SL, Stewart C, Mermel CH, Roberts SA, Kiezun A, Hammerman PS, McKenna A, Drier Y, Zou L, Ramos AH, Pugh TJ, Stransky N, Helman E, Kim J, Sougnez C, Ambrogio L, Nickerson E, Shefler E, Cortes ML, Auclair D, Saksena G, Voet D, Noble M, DiCara D, Lin P, Lichtenstein L, Heiman I, Fennell T, Imielinski M, Hernandez B, Hodis E, Baca S, Dulak AM, Lohr J, Landau DA, Wu J, Melendez-Zajgla J, Hidalgo-Miranda A, Koren A, McCarroll SA, Mora J, Crompton B, Onofrio R, Parkin M, Winckler W, Ardlie K, Gabriel SB, Roberts CWM, Biegel JA, Stegmaier K, Bass AJ, Garraway LA, Meyerson M, Golub TR, Gordenin DA, Sunyaev S, Lander ES, Getz G, 2013. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature 499, 214–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Lee JH, Lim YS, Yeon JE, Song TJ, Yu SJ, Gwak GY, Kim KM, Kim YJ, Lee JW, Yoon JH, 2015. Adjuvant immunotherapy with autologous cytokine-induced killer cells for hepatocellular carcinoma. Gastroenterology 148, 1383–1391 e1386. [DOI] [PubMed] [Google Scholar]
- Lee JS, 2015. The mutational landscape of hepatocellular carcinoma. Clin Mol Hepatol 21, 220–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leen AM, Sukumaran S, Watanabe N, Mohammed S, Keirnan J, Yanagisawa R, Anurathapan U, Rendon D, Heslop HE, Rooney CM, Brenner MK, Vera JF, 2014. Reversal of tumor immune inhibition using a chimeric cytokine receptor. Mol Ther 22, 1211–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Li N, Zhang Y, Fu H, Torres MB, Wang Q, Greten TF, Ho M, 2018. Abstract 2549: Development of CAR T-cell therapy targeting glypican-3 in liver cancer. Cancer Res. 78, 2549–2549. [Google Scholar]
- Li J, Stagg NJ, Johnston J, Harris MJ, Menzies SA, DiCara D, Clark V, Hristopoulos M, Cook R, Slaga D, Nakamura R, McCarty L, Sukumaran S, Luis E, Ye Z, Wu TD, Sumiyoshi T, Danilenko D, Lee GY, Totpal K, Ellerman D, Hotzel I, James JR, Junttila TT, 2017a. Membrane-proximal epitope facilitates efficient T cell synapse formation by anti-FcRH5/CD3 and is a requirement for myeloma cell killing. Cancer Cell 31, 383–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li LP, Lampert JC, Chen X, Leitao C, Popovic J, Muller W, Blankenstein T, 2010. Transgenic mice with a diverse human T cell antigen receptor repertoire. Nat Med 16, 1029–1034. [DOI] [PubMed] [Google Scholar]
- Li W, Guo L, Rathi P, Marinova E, Gao X, Wu MF, Liu H, Dotti G, Gottschalk S, Metelitsa LS, Heczey A, 2017b. Redirecting T Cells to Glypican-3 with 4-1BB Zeta Chimeric Antigen Receptors Results in Th1 Polarization and Potent Antitumor Activity. Hum Gene Ther 28, 437–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Moysey R, Molloy PE, Vuidepot AL, Mahon T, Baston E, Dunn S, Liddy N, Jacob J, Jakobsen BK, Boulter JM, 2005. Directed evolution of human T-cell receptors with picomolar affinities by phage display. Nat Biotechnol 23, 349–354. [DOI] [PubMed] [Google Scholar]
- Linette GP, Stadtmauer EA, Maus MV, Rapoport AP, Levine BL, Emery L, Litzky L, Bagg A, Carreno BM, Cimino PJ, Binder-Scholl GK, Smethurst DP, Gerry AB, Pumphrey NJ, Bennett D, Brewer JE, Dukes J, Harper J, Tayton-Martin HK, Jakobsen BK, Hassan NJ, Kalos M, June CH, 2013. Cardiovascular toxicity and titin cross-reactivity of affinity-enhanced T cells in myeloma and melanoma. Blood 122, 863–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Xu Y, Xiang J, Long L, Green S, Yang Z, Zimdahl B, Lu J, Cheng N, Horan LH, Liu B, Yan S, Wang P, Diaz J, Jin L, Nakano Y, Morales JF, Zhang P, Liu LX, Staley BK, Priceman SJ, Brown CE, Forman SJ, Chan VW, Liu C, 2017. Targeting Alpha-Fetoprotein (AFP)-MHC Complex with CAR T-Cell Therapy for Liver Cancer. Clin Cancer Res 23, 478–488. [DOI] [PubMed] [Google Scholar]
- Liu X, Jiang S, Fang C, Yang S, Olalere D, Pequignot EC, Cogdill AP, Li N, Ramones M, Granda B, Zhou L, Loew A, Young RM, June CH, Zhao Y, 2015. Affinity-Tuned ErbB2 or EGFR Chimeric Antigen Receptor T Cells Exhibit an Increased Therapeutic Index against Tumors in Mice. Cancer Res 75, 3596–3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Di S, Shi B, Zhang H, Wang Y, Wu X, Luo H, Wang H, Li Z, Jiang H, 2019. Armored Inducible Expression of IL-12 Enhances Antitumor Activity of Glypican-3-Targeted Chimeric Antigen Receptor-Engineered T Cells in Hepatocellular Carcinoma. J Immunol 203, 198–207. [DOI] [PubMed] [Google Scholar]
- Liu Y, Peng Y, Mi M, Guevara-Patino J, Munn DH, Fu N, He Y, 2009. Lentivector immunization stimulates potent CD8 T cell responses against melanoma self-antigen tyrosinase-related protein 1 and generates antitumor immunity in mice. J Immunol 182, 5960–5969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long AH, Haso WM, Shern JF, Wanhainen KM, Murgai M, Ingaramo M, Smith JP, Walker AJ, Kohler ME, Venkateshwara VR, Kaplan RN, Patterson GH, Fry TJ, Orentas RJ, Mackall CL, 2015. 4-1BB costimulation ameliorates T cell exhaustion induced by tonic signaling of chimeric antigen receptors. Nat Med 21, 581–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis CU, Savoldo B, Dotti G, Pule M, Yvon E, Myers GD, Rossig C, Russell HV, Diouf O, Liu E, Liu H, Wu MF, Gee AP, Mei Z, Rooney CM, Heslop HE, Brenner MK, 2011. Antitumor activity and long-term fate of chimeric antigen receptor-positive T cells in patients with neuroblastoma. Blood 118, 6050–6056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo G, Huang S, Xie X, Stockert E, Chen YT, Kubuschok B, Pfreundschuh M, 2002. Expression of cancer-testis genes in human hepatocellular carcinomas. Cancer Immun 2, 11. [PubMed] [Google Scholar]
- Lynn RC, Weber EW, Gennert D, Sotillo E, Xu P, Good Z, Anbunathan H, Jones R, Tieu V, Granja J, DeBourcy C, Majzner R, Satpathy AT, Quake SR, Chang H, Mackall CL, 2019. c-Jun overexpressing CAR-T cells are exhaustion-resistant and mediate enhanced antitumor activity. bioRxiv, 653725. [Google Scholar]
- Ma L, Dichwalkar T, Chang JYH, Cossette B, Garafola D, Zhang AQ, Fichter M, Wang C, Liang S, Silva M, Kumari S, Mehta NK, Abraham W, Thai N, Li N, Wittrup KD, Irvine DJ, 2019. Enhanced CAR-T cell activity against solid tumors by vaccine boosting through the chimeric receptor. Science 365, 162–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makarova-Rusher OV, Medina-Echeverz J, Duffy AG, Greten TF, 2015. The yin and yang of evasion and immune activation in HCC. J Hepatol 62, 1420–1429. [DOI] [PubMed] [Google Scholar]
- Martinez M, Moon EK, 2019. CAR T Cells for solid tumors: new strategies for finding, infiltrating, and surviving in the tumor microenvironment. Front Immunol 10, 128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruta M, Ochi T, Tanimoto K, Azuma T, Fujiwara H, Yasukawa M, 2017. Development of T-cell therapy by exploiting modified antibodies specific for A2/NY-ESO-1 for refractory myeloma. Blood 130, 1913–1913. [Google Scholar]
- Mestermann K, Giavridis T, Weber J, Rydzek J, Frenz S, Nerreter T, Mades A, Sadelain M, Einsele H, Hudecek M, 2019. The tyrosine kinase inhibitor dasatinib acts as a pharmacologic on/off switch for CAR T cells. Sci Transl Med 11, 11, pii: eaau5907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milone MC, Fish JD, Carpenito C, Carroll RG, Binder GK, Teachey D, Samanta M, Lakhal M, Gloss B, Danet-Desnoyers G, Campana D, Riley JL, Grupp SA, June CH, 2009. Chimeric receptors containing CD137 signal transduction domains mediate enhanced survival of T cells and increased antileukemic efficacy in vivo. Mol Ther 17, 1453–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizukoshi E, Kaneko S, 2019. Immune cell therapy for hepatocellular carcinoma. J Hematol Oncol 12, 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizukoshi E, Nakagawa H, Kitahara M, Yamashita T, Arai K, Sunagozaka H, Fushimi K, Kobayashi E, Kishi H, Muraguchi A, Kaneko S, 2015. Immunological features of T cells induced by human telomerase reverse transcriptase-derived peptides in patients with hepatocellular carcinoma. Cancer Lett 364, 98–105. [DOI] [PubMed] [Google Scholar]
- Mizukoshi E, Nakamoto Y, Marukawa Y, Arai K, Yamashita T, Tsuji H, Kuzushima K, Takiguchi M, Kaneko S, 2006a. Cytotoxic T cell responses to human telomerase reverse transcriptase in patients with hepatocellular carcinoma. Hepatology 43, 1284–1294. [DOI] [PubMed] [Google Scholar]
- Mizukoshi E, Nakamoto Y, Tsuji H, Yamashita T, Kaneko S, 2006b. Identification of alpha-fetoprotein-derived peptides recognized by cytotoxic T lymphocytes in HLA-A24+ patients with hepatocellular carcinoma. Int J Cancer 118, 1194–1204. [DOI] [PubMed] [Google Scholar]
- Morgan RA, Yang JC, Kitano M, Dudley ME, Laurencot CM, Rosenberg SA, 2010. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther 18, 843–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita D, Nishio N, Saito S, Tanaka M, Kawashima N, Okuno Y, Suzuki S, Matsuda K, Maeda Y, Wilson MH, Dotti G, Rooney CM, Takahashi Y, Nakazawa Y, 2018. Enhanced expression of anti-CD19 chimeric antigen receptor in piggyBac transposon-engineered T cells. Mol Ther Methods Clin Dev 8, 131–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa H, Mizukoshi E, Kobayashi E, Tamai T, Hamana H, Ozawa T, Kishi H, Kitahara M, Yamashita T, Arai K, Terashima T, Iida N, Fushimi K, Muraguchi A, Kaneko S, 2017. Association between high-avidity T-cell receptors, induced by alpha-fetoprotein-derived peptides, and anti-tumor effects in patients with hepatocellular carcinoma. Gastroenterology 152, 1395–1406 e1310. [DOI] [PubMed] [Google Scholar]
- Nakatsura T, Kageshita T, Ito S, Wakamatsu K, Monji M, Ikuta Y, Senju S, Ono T, Nishimura Y, 2004a. Identification of glypican-3 as a novel tumor marker for melanoma. Clin Cancer Res 10, 6612–6621. [DOI] [PubMed] [Google Scholar]
- Nakatsura T, Komori H, Kubo T, Yoshitake Y, Senju S, Katagiri T, Furukawa Y, Ogawa M, Nakamura Y, Nishimura Y, 2004b. Mouse homologue of a novel human oncofetal antigen, glypican-3, evokes T-cell-mediated tumor rejection without autoimmune reactions in mice. Clin Cancer Res 10, 8630–8640. [DOI] [PubMed] [Google Scholar]
- Obenaus M, Leitao C, Leisegang M, Chen X, Gavvovidis I, van der Bruggen P, Uckert W, Schendel DJ, Blankenstein T, 2015. Identification of human T-cell receptors with optimal affinity to cancer antigens using antigen-negative humanized mice. Nat Biotechnol 33, 402–407. [DOI] [PubMed] [Google Scholar]
- Orentas RJ, Sindiri S, Duris C, Wen X, He J, Wei JS, Jarzembowski J, Khan J, 2017. Paired Expression Analysis of Tumor Cell Surface Antigens. Front Oncol 7, 173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott PA, Hu Z, Keskin DB, Shukla SA, Sun J, Bozym DJ, Zhang W, Luoma A, Giobbie-Hurder A, Peter L, Chen C, Olive O, Carter TA, Li S, Lieb DJ, Eisenhaure T, Gjini E, Stevens J, Lane WJ, Javeri I, Nellaiappan K, Salazar AM, Daley H, Seaman M, Buchbinder EI, Yoon CH, Harden M, Lennon N, Gabriel S, Rodig SJ, Barouch DH, Aster JC, Getz G, Wucherpfennig K, Neuberg D, Ritz J, Lander ES, Fritsch EF, Hacohen N, Wu CJ, 2017. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature 547, 217–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer DH, Midgley RS, Mirza N, Torr EE, Ahmed F, Steele JC, Steven NM, Kerr DJ, Young S, Adams DH, 2009. A phase II study of adoptive immunotherapy using dendritic cells pulsed with tumor lysate in patients with hepatocellular carcinoma. Hepatology 49, 124–132. [DOI] [PubMed] [Google Scholar]
- Paszkiewicz PJ, Frassle SP, Srivastava S, Sommermeyer D, Hudecek M, Drexler I, Sadelain M, Liu L, Jensen MC, Riddell SR, Busch DH, 2016. Targeted antibody-mediated depletion of murine CD19 CAR T cells permanently reverses B cell aplasia. J Clin Invest 126, 4262–4272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pez F, Lopez A, Kim M, Wands JR, Caron de Fromentel C, Merle P, 2013. Wnt signaling and hepatocarcinogenesis: molecular targets for the development of innovative anticancer drugs. J Hepatol 59, 1107–1117. [DOI] [PubMed] [Google Scholar]
- Philip M, Fairchild L, Sun L, Horste EL, Camara S, Shakiba M, Scott AC, Viale A, Lauer P, Merghoub T, Hellmann MD, Wolchok JD, Leslie CS, Schietinger A, 2017. Chromatin states define tumour-specific T cell dysfunction and reprogramming. Nature 545, 452–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prieto J, Melero I, Sangro B, 2015. Immunological landscape and immunotherapy of hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol 12, 681–700. [DOI] [PubMed] [Google Scholar]
- Qasim W, Brunetto M, Gehring AJ, Xue SA, Schurich A, Khakpoor A, Zhan H, Ciccorossi P, Gilmour K, Cavallone D, Moriconi F, Farzhenah F, Mazzoni A, Chan L, Morris E, Thrasher A, Maini MK, Bonino F, Stauss H, Bertoletti A, 2015. Immunotherapy of HCC metastases with autologous T cell receptor redirected T cells, targeting HBsAg in a liver transplant patient. J Hepatol 62, 486–491. [DOI] [PubMed] [Google Scholar]
- Rafiq S, Purdon TJ, Daniyan AF, Koneru M, Dao T, Liu C, Scheinberg DA, Brentjens RJ, 2017. Optimized T-cell receptor-mimic chimeric antigen receptor T cells directed toward the intracellular Wilms Tumor 1 antigen. Leukemia 31, 1788–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport AP, Stadtmauer EA, Binder-Scholl GK, Goloubeva O, Vogl DT, Lacey SF, Badros AZ, Garfall A, Weiss B, Finklestein J, Kulikovskaya I, Sinha SK, Kronsberg S, Gupta M, Bond S, Melchiori L, Brewer JE, Bennett AD, Gerry AB, Pumphrey NJ, Williams D, Tayton-Martin HK, Ribeiro L, Holdich T, Yanovich S, Hardy N, Yared J, Kerr N, Philip S, Westphal S, Siegel DL, Levine BL, Jakobsen BK, Kalos M, June CH, 2015. NY-ESO-1-specific TCR-engineered T cells mediate sustained antigen-specific antitumor effects in myeloma. Nat Med 21, 914–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richman SA, Nunez-Cruz S, Moghimi B, Li LZ, Gershenson ZT, Mourelatos Z, Barrett DM, Grupp SA, Milone MC, 2018. High-affinity GD2-specific CAR T cells induce fatal encephalitis in a preclinical neuroblastoma model. Cancer Immunol Res 6, 36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, Lee W, Yuan J, Wong P, Ho TS, Miller ML, Rekhtman N, Moreira AL, Ibrahim F, Bruggeman C, Gasmi B, Zappasodi R, Maeda Y, Sander C, Garon EB, Merghoub T, Wolchok JD, Schumacher TN, Chan TA, 2015. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 348, 124–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins PF, Kassim SH, Tran TL, Crystal JS, Morgan RA, Feldman SA, Yang JC, Dudley ME, Wunderlich JR, Sherry RM, Kammula US, Hughes MS, Restifo NP, Raffeld M, Lee CC, Li YF, El-Gamil M, Rosenberg SA, 2015. A pilot trial using lymphocytes genetically engineered with an NY-ESO-1-reactive T-cell receptor: long-term follow-up and correlates with response. Clin Cancer Res 21, 1019–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins PF, Li YF, El-Gamil M, Zhao Y, Wargo JA, Zheng Z, Xu H, Morgan RA, Feldman SA, Johnson LA, Bennett AD, Dunn SM, Mahon TM, Jakobsen BK, Rosenberg SA, 2008. Single and dual amino acid substitutions in TCR CDRs can enhance antigen-specific T cell functions. J Immunol 180, 6116–6131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosati SF, Parkhurst MR, Hong Y, Zheng Z, Feldman SA, Rao M, Abate-Daga D, Beard RE, Xu H, Black MA, Robbins PF, Schrump DA, Rosenberg SA, Morgan RA, 2014. A novel murine T-cell receptor targeting NY-ESO-1. J Immunother 37, 135–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeidi A, Zandi K, Cheok YY, Saeidi H, Wong WF, Lee CYQ, Cheong HC, Yong YK, Larsson M, Shankar EM, 2018. T-cell exhaustion in chronic infections: reversing the state of exhaustion and reinvigorating optimal protective immune responses. Front Immunol 9, 2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahin U, Derhovanessian E, Miller M, Kloke BP, Simon P, Lower M, Bukur V, Tadmor AD, Luxemburger U, Schrors B, Omokoko T, Vormehr M, Albrecht C, Paruzynski A, Kuhn AN, Buck J, Heesch S, Schreeb KH, Muller F, Ortseifer I, Vogler I, Godehardt E, Attig S, Rae R, Breitkreuz A, Tolliver C, Suchan M, Martic G, Hohberger A, Sorn P, Diekmann J, Ciesla J, Waksmann O, Bruck AK, Witt M, Zillgen M, Rothermel A, Kasemann B, Langer D, Bolte S, Diken M, Kreiter S, Nemecek R, Gebhardt C, Grabbe S, Holler C, Utikal J, Huber C, Loquai C, Tureci O, 2017. Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature 547, 222–226. [DOI] [PubMed] [Google Scholar]
- Sawada Y, Yoshikawa T, Nobuoka D, Shirakawa H, Kuronuma T, Motomura Y, Mizuno S, Ishii H, Nakachi K, Konishi M, Nakagohri T, Takahashi S, Gotohda N, Takayama T, Yamao K, Uesaka K, Furuse J, Kinoshita T, Nakatsura T, 2012. Phase I trial of a glypican-3-derived peptide vaccine for advanced hepatocellular carcinoma: immunologic evidence and potential for improving overall survival. Clin Cancer Res 18, 3686–3696. [DOI] [PubMed] [Google Scholar]
- Schmueck-Henneresse M, Omer B, Shum T, Tashiro H, Mamonkin M, Lapteva N, Sharma S, Rollins L, Dotti G, Reinke P, Volk HD, Rooney CM, 2017. Comprehensive approach for identifying the T cell subset origin of CD3 and CD28 antibody-activated chimeric antigen receptor-modified T cells. J Immunol 199, 348–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze K, Imbeaud S, Letouze E, Alexandrov LB, Calderaro J, Rebouissou S, Couchy G, Meiller C, Shinde J, Soysouvanh F, Calatayud AL, Pinyol R, Pelletier L, Balabaud C, Laurent A, Blanc JF, Mazzaferro V, Calvo F, Villanueva A, Nault JC, Bioulac-Sage P, Stratton MR, Llovet JM, Zucman-Rossi J, 2015. Exome sequencing of hepatocellular carcinomas identifies new mutational signatures and potential therapeutic targets. Nat Genet 47, 505–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo H, Chen J, Gonzalez-Avalos E, Samaniego-Castruita D, Das A, Wang YH, Lopez-Moyado IF, Georges RO, Zhang W, Onodera A, Wu CJ, Lu LF, Hogan PG, Bhandoola A, Rao A, 2019. TOX and TOX2 transcription factors cooperate with NR4A transcription factors to impose CD8+ T cell exhaustion. Proc Natl Acad Sci U S A 116, 12410–12415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu Y, Suzuki T, Yoshikawa T, Tsuchiya N, Sawada Y, Endo I, Nakatsura T, 2018. Cancer immunotherapy-targeted glypican-3 or neoantigens. Cancer Sci 109, 531–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh H, Figliola MJ, Dawson MJ, Olivares S, Zhang L, Yang G, Maiti S, Manuri P, Senyukov V, Jena B, Kebriaei P, Champlin RE, Huls H, Cooper LJ, 2013. Manufacture of clinical-grade CD19-specific T cells stably expressing chimeric antigen receptor using Sleeping Beauty system and artificial antigen presenting cells. PLoS One 8, e64138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommermeyer D, Hudecek M, Kosasih PL, Gogishvili T, Maloney DG, Turtle CJ, Riddell SR, 2016. Chimeric antigen receptor-modified T cells derived from defined CD8+ and CD4+ subsets confer superior antitumor reactivity in vivo. Leukemia 30, 492–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear TT, Callender GG, Roszkowski JJ, Moxley KM, Simms PE, Foley KC, Murray DC, Scurti M, Li M, Thomas JT, Langerman A, Garrett-Mayer E, Zhang Y, Nishimura MI, 2016. TCR gene-modified T cells can efficiently treat established hepatitis C-associated hepatocellular carcinoma tumors. Cancer Immunol Immunother 65, 293–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava S, Riddell SR, 2015. Engineering CAR-T cells: Design concepts. Trends Immunol 36, 494–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadtmauer EA, Faitg TH, Lowther DE, Badros AZ, Chagin K, Dengel K, Iyengar M, Melchiori L, Navenot JM, Norry E, Trivedi T, Wang R, Binder GK, Amado R, Rapoport AP, 2019. Long-term safety and activity of NY-ESO-1 SPEAR T cells after autologous stem cell transplant for myeloma. Blood Adv 3, 2022–2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoiber S, Cadilha BL, Benmebarek MR, Lesch S, Endres S, Kobold S, 2019. Limitations in the design of chimeric antigen receptors for cancer Therapy. Cells 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suryadevara CM, Desai R, Farber SH, Choi BD, Swartz AM, Shen SH, Gedeon PC, Snyder DJ, Herndon JE 2nd, Healy P, Reap EA, Archer GE, Fecci PE, Sampson JH, Sanchez-Perez L, 2019. Preventing Lck activation in CAR T cells confers Treg resistance but requires 4-1BB signaling for them to persist and treat solid tumors in nonlymphodepleted hosts. Clin Cancer Res 25, 358–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turtle CJ, Hanafi LA, Berger C, Gooley TA, Cherian S, Hudecek M, Sommermeyer D, Melville K, Pender B, Budiarto TM, Robinson E, Steevens NN, Chaney C, Soma L, Chen X, Yeung C, Wood B, Li D, Cao J, Heimfeld S, Jensen MC, Riddell SR, Maloney DG, 2016a. CD19 CAR-T cells of defined CD4+:CD8+ composition in adult B cell ALL patients. J Clin Invest 126, 2123–2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turtle CJ, Hanafi LA, Berger C, Hudecek M, Pender B, Robinson E, Hawkins R, Chaney C, Cherian S, Chen X, Soma L, Wood B, Li D, Heimfeld S, Riddell SR, Maloney DG, 2016b. Immunotherapy of non-Hodgkin’s lymphoma with a defined ratio of CD8+ and CD4+ CD19−specific chimeric antigen receptor-modified T cells. Sci Transl Med 8, 355ra116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Aguilar B, Starr R, Alizadeh D, Brito A, Sarkissian A, Ostberg JR, Forman SJ, Brown CE, 2018. Glioblastoma-targeted CD4+ CAR T cells mediate superior antitumor activity. JCI Insight 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Berger C, Wong CW, Forman SJ, Riddell SR, Jensen MC, 2011. Engraftment of human central memory-derived effector CD8+ T cells in immunodeficient mice. Blood 117, 1888–1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Jiang H, Luo H, Sun Y, Shi B, Sun R, Li Z, 2019. An IL-4/21 Inverted Cytokine Receptor Improving CAR-T Cell Potency in Immunosuppressive Solid-Tumor Microenvironment. Front Immunol 10, 1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welzel TM, Graubard BI, Quraishi S, Zeuzem S, Davila JA, El-Serag HB, McGlynn KA, 2013. Population-attributable fractions of risk factors for hepatocellular carcinoma in the United States. Am J Gastroenterol 108, 1314–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wherry EJ, 2011. T cell exhaustion. Nat Immunol 12, 492–499. [DOI] [PubMed] [Google Scholar]
- Wu CY, Roybal KT, Puchner EM, Onuffer J, Lim WA, 2015. Remote control of therapeutic T cells through a small molecule-gated chimeric receptor. Science 350, aab4077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong W, Chen Y, Kang X, Chen Z, Zheng P, Hsu YH, Jang JH, Qin L, Liu H, Dotti G, Liu D, 2018. Immunological synapse predicts effectiveness of chimeric antigen receptor cells. Mol Ther 26, 963–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Dotti G, 2016. Selection bias: maintaining less-differentiated T cells for adoptive immunotherapy. J Clin Invest 126, 35–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Yang Z, Horan LH, Zhang P, Liu L, Zimdahl B, Green S, Lu J, Morales JF, Barrett DM, Grupp SA, Chan VW, Liu H, Liu C, 2018. A novel antibody-TCR (AbTCR) platform combines Fab-based antigen recognition with gamma/delta-TCR signaling to facilitate T-cell cytotoxicity with low cytokine release. Cell Discov 4, 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Zhang M, Ramos CA, Durett A, Liu E, Dakhova O, Liu H, Creighton CJ, Gee AP, Heslop E, Rooney CM, Savoldo B, Dotti G, 2014. Closely related T-memory stem cells correlate with in vivo expansion of CAR.CD19-T cells and are preserved by IL-7 and IL-15. Blood 123, 3750–3759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L, Liu B, 2019. Critical factors in chimeric antigen receptor-modified T-cell (CAR-T) therapy for solid tumors. Onco Targets Ther 12, 193–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yáñez L, Sánchez-Escamilla M, & Perales MA , 2019. CAR T cell toxicity: current management and future directions. HemaSphere 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Kohler ME, Chien CD, Sauter CT, Jacoby E, Yan C, Hu Y, Wanhainen K, Qin H, Fry TJ, 2017. TCR engagement negatively affects CD8 but not CD4 CAR T cell expansion and leukemic clearance. Sci Transl Med 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao S, Johnson C, Hu Q, Yan L, Liu B, Ambrosone CB, Wang J, Liu S, 2016. Differences in somatic mutation landscape of hepatocellular carcinoma in Asian American and European American populations. Oncotarget 7, 40491–40499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M, Luo H, Fan M, Wu X, Shi B, Di S, Liu Y, Pan Z, Jiang H, Li Z, 2018. Development of GPC3-Specific Chimeric Antigen Receptor-Engineered Natural Killer Cells for the Treatment of Hepatocellular Carcinoma. Mol Ther 26, 366–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang HH, Mei MH, Fei R, Liao WJ, Wang XY, Qin LL, Wang JH, Wei L, Chen HS, 2010. Regulatory T cell depletion enhances tumor specific CD8 T-cell responses, elicited by tumor antigen NY-ESO-1b in hepatocellular carcinoma patients, in vitro. Int J Oncol 36, 841–848. [DOI] [PubMed] [Google Scholar]
- Zhang J, Wang L, 2019. The Emerging World of TCR-T Cell Trials Against Cancer: A Systematic Review. Technol Cancer Res Treat 18, 1533033819831068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T, Wu MR, Sentman CL, 2012. An NKp30-based chimeric antigen receptor promotes T cell effector functions and antitumor efficacy in vivo. J Immunol 189, 2290–2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z, Condomines M, van der Stegen SJC, Perna F, Kloss CC, Gunset G, Plotkin J, Sadelain M, 2015. Structural design of Engineered Costimulation Determines Tumor Rejection Kinetics and Persistence of CAR T Cells. Cancer Cell 28, 415–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Xiao H, Liu Y, Peng Y, Hong Y, Yagita H, Chandler P, Munn DH, Mellor A, Fu N, He Y, 2010. Blockade of programmed death-1 pathway rescues the effector function of tumor-infiltrating T cells and enhances the antitumor efficacy of lentivector immunization. J Immunol 185, 5082–5092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Zhu H, Lu J, 2015. PTEN and hTERT gene expression and the correlation with human hepatocellular carcinoma. Pathol Res Pract 211, 316–319. [DOI] [PubMed] [Google Scholar]
- Zhu W, Peng Y, Wang L, Hong Y, Jiang X, Li Q, Liu H, Huang L, Wu J, Celis E, Merchen T, Kruse E, He Y, 2018. Identification of alpha-fetoprotein-specific T-cell receptors for hepatocellular carcinoma immunotherapy. Hepatology 68, 574–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucman-Rossi J, Villanueva A, Nault JC, Llovet JM, 2015. Genetic landscape and biomarkers of hepatocellular carcinoma. Gastroenterology 149, 1226–1239 e1224. [DOI] [PubMed] [Google Scholar]