Abstract
The anticoagulation field is experiencing a renaissance that began with regulatory approval of the direct thrombin inhibitor, dabigatran, a Direct Oral Anticoagulant (DOAC) in 2010. The DOAC medication class has rapidly evolved to include the additional approval of four direct factor Xa inhibitors. Commensurately, DOAC utilization has grown and collectively account for the majority of new anticoagulant prescriptions. Despite exclusion of moderate-to-severe kidney disease patients from most DOAC pivotal trials, DOACs are increasingly utilized in this setting. An advantage of DOACs is similar or improved antithrombotic efficacy with less bleeding risk (when compared to traditional agents). Several post-hoc analyses, retrospective studies, claims data studies, and meta-analyses suggest that these benefits extend to kidney disease patients. However, the lack of randomized controlled trial data in specific kidney disease settings, with their unique pathophysiology, should be a call-to-action for the kidney community to systematically study these agents; especially since early data suggest that DOACs may pose less risk of anticoagulant-related nephropathy than vitamin K antagonists. Most DOACs are renally cleared and are significantly protein bound in circulation, thus the pharmacokinetics of these drugs are influenced by reduced renal function and proteinuria. DOACs are susceptible to altered metabolism by P-glycoprotein inhibitors and inducers, including drugs commonly utilized for management of kidney disease co-morbidities. We summarize the currently available literature on DOAC use in kidney disease and illustrate knowledge gaps which represent important opportunities for prospective investigation.
Keywords: Anticoagulation, Chronic Kidney Disease, End-Stage Kidney Disease, Nephrotic Syndrome, Lupus Nephritis, Dialysis, Atrial Fibrillation, Venous Thromboembolism
INTRODUCTION
Kidney disease patients are at increased risk for both thrombotic disease and bleeding events, thus requiring careful clinical attention to hemostatic balance.1–4 Arterial thromboembolic disease arises primarily from co-morbid atherosclerosis and its management in kidney disease patients has been recently reviewed in this journal and elsewhere.1, 5–10 With the exception of stroke prevention in patients with co-morbid atrial fibrillation (AF), arterial thrombosis is primarily managed with antiplatelet agents, although recent data support the use of low-dose rivaroxaban (2.5 mg twice daily) in combination with antiplatelet therapy for coronary or peripheral artery disease.11, 12 In addition to other reviews, antiplatelet agent use in kidney disease scenarios has been a recent Cochrane review subject.13–16 Meanwhile, there have been significant advancements in oral anticoagulant medications for primary stroke prevention in AF and secondary prophylaxis in venous thromboembolic (VTE) disease. This review will focus on the use of oral anticoagulant medications for kidney disease patients with an emphasis on the Direct Oral Anticoagulants (DOACs).
The prevalence of AF in chronic kidney disease (CKD) patients is 2–3–fold higher than in the general population and an estimated 7–20% of end-stage kidney disease (ESKD) patients have AF.17–24 In a large Danish case-control study, the odds ratio (OR) for VTE in adults with kidney disease was 1.41–2.89.2 Our group recently analyzed United States claims data demonstrating ~1:200 children with chronic renal disease suffer from VTE complications (vs. ~1:10,000 in the general pediatric population).3, 25 Nephrologists are likely to encounter patients requiring anticoagulation for these or other indications during routine practice.
Whereas vitamin K antagonists (VKA) and heparins served as the mainstays of anticoagulation for decades, the field is evolving rapidly. Five DOACs have received regulatory approval and additional novel anticoagulant approaches are presently in clinical trials. We will thus review the pharmacology of approved anticoagulants and their use in various kidney disease contexts.
ANTICOAGULATION PHARMACOLOGY
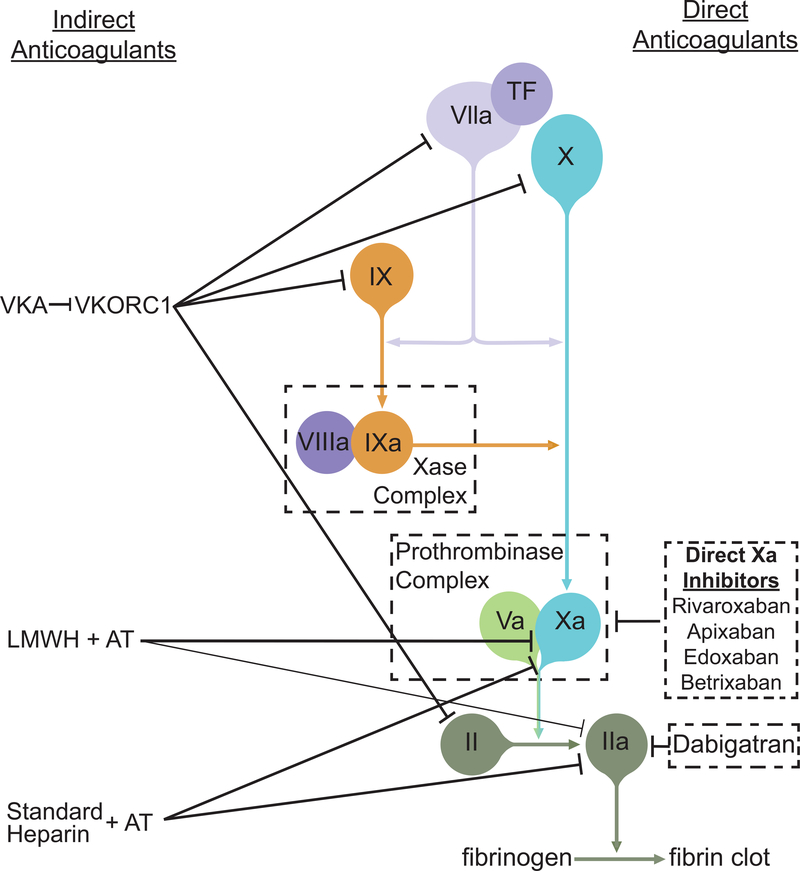
Heparins are indirect anticoagulants that potentiate the enzymatic activity of antithrombin (AT; Figure).26 This activity is dependent upon interaction of a specific heparin pentasaccharide sequence with AT. Whereas standard (unfractionated) heparin effectively inhibits both thrombin and factor Xa, low molecular weight heparins (LMWH; e.g. enoxaparin, dalteparin) are less potent thrombin antagonists, exerting their effects predominantly via factor Xa inhibition. The synthetically produced pentasaccharide (fondaparinux) is factor Xa selective. Heparinoids (e.g. danaparoid) are mixtures of heparan, dermatan, and chondroitin sulfates that primarily antagonize factor Xa with less prominent inhibition of other coagulation factors. Heparins, pentasaccharides, and heparinoids are administered either intravenously (standard heparin) or subcutaneously (all compounds). Standard heparin is eliminated by non-renal metabolism, whereas LMWHs and heparinoids are cleared renally and non-renally. While standard heparin needs no dose adjustment for renal insufficiency, the others should be dose adjusted.
Figure:
Schematic Representation of Oral Anticoagulant Mechanisms of Action
VKAs (e.g. warfarin, acenocoumarol) are indirect anticoagulants which inhibit vitamin K epoxide reductase complex 1 (VKORC1). Consequently, the reduced form of vitamin K becomes depleted and unavailable to facilitate post-translational γ-carboxylation of factors II (prothrombin), VII, IX, and X. Because factor VII has the shortest half-life, the greatest effects are seen on the prothrombin time (PT) and VKAs are thus monitored with the PT-derived international normalized ratio (INR). Clearance is via hepatic metabolism primarily involving CYP2C9. The VKA therapeutic window is narrow and requires careful monitoring and frequent dose adjustment.27 Patients with chronic kidney disease tend to require lower maintenance doses than other patients and experience more volatile INRs, requiring more intense monitoring.28
Several parenteral direct anticoagulants have been clinically available since ~2000.29, 30 Argatroban is a competitive thrombin inhibitor.30 It is cleared hepatically via CYP3A4/5 metabolism and has a short half-life requiring continuous intravenous administration. Bivalirudin (a synthetic oligopeptide mimetic of the naturally occurring medicinal leech venom anticoagulant, hirudin) is a selective, reversible thrombin antagonist.31 Similar to argatroban, its short half-life requires continuous intravenous infusion. Desirudin, a recombinant hirudin analog is suitable for intermittent subcutaneous injection.32 Both bivalirudin and desirudin are predominantly cleared renally (50% as unchanged drug) and may require dose adjustment for renal impairment.
Originally referred to as “new,” “novel,” or “non-VKA oral anticoagulants” (NOACs), the preferred international consensus nomenclature for oral anticoagulants that directly inhibit a single molecular target is “direct oral anticoagulant” (DOAC).33 These agents have received regulatory approval on the basis of clinical trials demonstrating improved safety and non-inferiority, usually in comparison to warfarin. These agents have similar or improved efficacy in primary or secondary thromboprophylaxis and, perhaps more importantly, they generally have a superior safety profile (less clinically relevant major and non-major bleeding). The improved safety profile may be due to their more predictable pharmacokinetics over indirect anticoagulants (esp. VKAs) or result from their direct mechanisms of action. The first DOAC (dabigatran) received FDA approval in 2010. DOACs have since captured a growing share of anticoagulant prescriptions, recently surpassing warfarin in the United States and United Kingdom.34, 35
Dabigatran etexilate mesylate is an orally bioavailable prodrug requiring metabolism by both plasma and hepatic esterases to dabigatran, the active drug.36, 37 Dabigatran is currently the only clinically-approved direct thrombin inhibitor which binds reversibly to the active site of thrombin (Figure). Its clearance is predominantly renal (80% as dabigatran or active metabolites; Table 1).37–40 In contrast, there are currently four approved factor Xa-antagonist DOACs: rivaroxaban, apixaban, edoxaban, and betrixaban. These drugs all bind reversibly to the factor Xa active site and dampen downstream thrombin and fibrin generation.41 However, these compounds vary considerably in their pharmacokinetics, metabolism, and renal clearance (Table 1).38 Rivaroxaban has the shortest half-life (5–9 h), apixaban and edoxaban are intermediate (10–14 h), and betrixaban the longest (19–27 h). Rivaroxaban and apixaban undergo hepatic metabolism (primarily CYP3A4/5) to inactive metabolites whereas edoxaban and betrixaban undergo minimal hepatic metabolism. Rivaroxaban excretion is predominantly urinary (66% by active tubular excretion; 36% as unchanged drug). Apixaban is also excreted in urine (27% as parent drug). Edoxaban is about 50% renally cleared (primarily as unchanged drug) whereas betrixaban is predominantly (85%) excreted in the feces.
Table 1:
Pharmacology of Direct Oral Anticoagulants+
| Dabigatran | Rivaroxaban | Apixaban | Edoxaban | Betrixaban | |
|---|---|---|---|---|---|
| Target | Thrombin | Factor Xa | Factor Xa | Factor Xa | Factor Xa |
| Half-Life (h) | 12–17 | 5–9 | 12 | 10–14 | 19–27 |
| Renal Excretion (%) | 80^ | 66 | 27 | 50 | 11 |
| Fecal Excretion (%) | Not Applicable | 7 | ∼50 | 50 | 85 |
| Hepatic Metabolism | No | CYP3A4/5; CYP2J2 | CYP3A4/5; others | CYP3A4/5 (minimal) | Minimal |
| Protein Binding (%) | 35 | 92–95 | 87 | 55 | 60 |
| Dialyzable | Yes* | No | No | No | Not Studied |
| Reversal Agent | Idarucizumab | Andexanet Alfa | Andexanet Alfa | Andexanet Alfa?$ | Andexanet Alfa?$ |
Dabigatran is dialyzable (~57% removed over 4 hours), but rivaroxaban, apixaban, and edoxaban are not (betrixaban has not yet been studied). Reversal agents for the DOACs have recently been approved (Table 1). Idarucizumab, a monoclonal antibody fragment, binds dabigatran with an affinity 350-fold higher than thrombin, neutralizing the drug within minutes.42 Andexanet alfa, a recombinant, enzymatically inert mutant factor Xa molecule, competes with endogenous factor Xa for binding of factor Xa DOACs.43 Andexanet also restores hemostasis within minutes, but reversal may be incomplete in a substantial portion of patients. Moderate renal impairment (creatinine clearance (CrCl) 30–60 mL/min) modestly increases the half-life of idarucizumab which is excreted in the urine whereas andexanet has no measurable renal elimination.44 Renal dose adjustments are not recommended for either antidote.
ANTICOAGULATION SCENARIOS IN KIDNEY DISEASE
Kidney disease has been identified as a risk factor for both VTE and AF, two common indications for anticoagulation.1–3, 17–24 Although CKD patients were excluded from the pivotal, phase 3 DOAC trials. Data on their use, efficacy and side effects in various kidney diseases and levels of renal function are emerging from post-marketing studies. Below, we describe common causes of kidney disease and particular scenarios which might warrant anticoagulation (Table 2).45
Table 2:
DOAC Dosages by Common Indications, Renal Impairment, and Regulatory Agency Labeling†
| United States Food and Drug Administration (FDA) | European Medicines Agency (EMA) | |||
|---|---|---|---|---|
| Indication DOAC | CrCl* 15–29 mL/min | CrCl* 30–50 mL/min | CrCl* 15–29 mL/min | CrCl* 30–50 mL/min |
| Atrial Fibrillation | ||||
| Dabigatran | 75 mg twice daily | 150 mg twice daily | NR^ | 150 mg or 110 mg twice daily# |
| Rivaroxaban | 15 mg once daily | 15 mg once daily | 15 mg once daily | 15 mg once daily |
| Apixaban | 5 mg twice daily$ | 5 mg twice daily$ | 2.5 mg twice daily | 5 mg twice daily$ |
| Edoxaban | 30 mg once daily | 30 mg once daily | 30 mg once daily | 30 mg once daily |
| Betrixaban | NLα | NLα | NAβ | NAβ |
| VTE Treatment | ||||
| Dabigatran | NR^ | 150 mg twice daily+ | NR^ | 150 mg or 110 mg twice daily#+ |
| Rivaroxaban | Avoid Use | 15 mg twice daily x3 weeks, then 20 mg once daily | 15 mg twice daily x3 weeks, then 20 mg once dailyγ | 15 mg twice daily x3 weeks, then 20 mg once daily |
| Apixaban | 10 mg twice daily x7 days, then 5 mg twice daily | 10 mg twice daily x7 days, then 5 mg twice daily | 10 mg twice daily x7 days, then 5 mg twice daily | 10 mg twice daily x7 days, then 5 mg twice daily |
| Edoxaban | 30 mg once daily+ | 30 mg once daily+ | 30 mg once daily+ | 30 mg once daily+ |
| Betrixaban | NLα | NLα | NAβ | NAβ |
| VTE Prophylaxisδ | ||||
| Dabigatran | NR^ | 150 mg once daily | NR^ | 75 mg on day 1, then 150 mg once daily |
| Rivaroxaban | Avoid Use | 10 mg once daily | 10 mg once daily | 10 mg once daily |
| Apixaban | 2.5 mg twice daily | 2.5 mg twice daily | 2.5 mg twice daily | 2.5 mg twice daily |
| Edoxaban | NLα | NLα | NLα | NLα |
| Betrixabanδ | 80 mg on day 1, then 40 mg once daily | 160 mg on day 1, then 80 mg once daily | NAβ | NAβ |
Adapted from Parker and Thachil45 and respective US and European prescribing information.
CrCl: Creatinine clearance by the Cockcroft-Gault method.
NR: Not recommended.
Clinical judgement based upon individual patient bleeding vs. thrombotic risk.
Following an initial 5 day period of parenteral anticoagulation.
Dose adjusted to 2.5 mg twice daily if any 2 of the following criteria are met: serum creatinine >1.5 mg/dL (>133 μmol/L), weight <60 kg, or age >80 years.
NL: Not Labeled for this indication
NA: Not Applicable; Betrixaban is not approved by the EMA for any indication.
Consider dose reduction to 15 mg once daily.
Post-arthroplasy (Total Hip or Knee Replacement) prophylaxis for all drugs except betrixaban, which is FDA-approved for VTE prophylaxis in acutely ill medical patients.
Nephrotic Syndrome
VTE is a well-recognized nephrotic syndrome (NS) complication.46–48 In the aforementioned Danish registry, adult NS patients had the highest risk for VTE (OR 2.17 [95% CI 1.68–2.80]). Similarly our United States claims data analysis demonstrated that children with NS have the highest VTE prevalence (2% vs. 0.4%).2, 3 Reported NS-associated VTE prevalence varies dependent upon how VTE was ascertained and underlying pathology. In studies employing active VTE screening in adults with NS, the prevalence of renal vein thrombosis (RVT) was as high as 24% and highest in those with membranous nephropathy (MN), approaching 37%.47 In childhood NS, the overall prevalence is much lower (~3%), although the majority of these studies reported clinically evident VTE.47 Among children, those with MN and similar pathology demonstrated the highest VTE occurrence.49
In a cohort of 1,313 glomerular disease patients (MN, focal segmental glomerulosclerosis [FSGS], and IgA nephropathy [IgAN]), the MN group had the highest VTE risk, after disease severity adjustment (proteinuria and hypoalbuminemia), with a hazard ratio (HR) of 10.8 (vs. IgA nephropathy).50 Patients with FSGS were at intermediate risk (HR 5.9). Serum albumin was an independent VTE risk factor. In a pooled analysis of two registries including 898 MN patients, clinically evident VTE was noted in ~7%, and VTE risk increased with worsening hypoalbuminemia (highest when albumin <2.8 g/dL).51 Most VTE occurred within 2 years of diagnosis, consistent with other data suggesting that VTE is most likely early in the course of NS (sometimes prior or simultaneous to diagnosis).50, 51
Based on similar data, the 2012 KDIGO guidelines recommend consideration of prophylactic warfarin therapy for MN if serum albumin is <2.5 g/dL and additional thrombosis risk factors are present (grade 2C evidence).52 Based on the above data, a clinical guidance tool was developed to guide prophylactic warfarin use in MN patients while accounting for bleeding risk (https://www.med.unc.edu/gntools/).53 Generally, this tool suggests anticoagulation for MN patients with low bleeding risk and albumin <3 g/dL. However, this tool has limitations including a lack of validation studies. Because bleeding risk calculations in this algorithm are derived from warfarin safety data, the tool should only be applied to warfarin prophylaxis in adult MN cases. Optimal VTE prophylaxis for NS remains unclear as many studies have included heterogeneous causes of NS in which risk varies as described above. LMWH has been evaluated in an uncontrolled study in which no VTE events were noted among patients in the LMWH group.54 Another observational study compared 44 patients receiving prophylactic LMWH or warfarin to 35 control patients.55 Four VTEs were observed in the control group compared to none in the prophylaxis group. Two major bleeding events were reported in the prophylaxis group, both of whom were taking concomitant aspirin. There is no consensus on prophylaxis duration; however, most data suggest that hypercoagulopathy improves with NS remission.52 Thus, discontinuation of prophylaxis may be reasonable once sustained complete remission is achieved and after contemplating other patient-specific thrombotic and bleeding risks.
Data on DOAC use in NS are beginning to emerge. Apixaban use was recently described for two NS patients.56 One had minimal change disease and was treated with prophylactic apixaban until remission. The second had MN with a remote history of VTE and received apixaban prophylaxis for 3 months until resolution of NS. Neither patient experienced VTE, and the first had a minor epistaxis episode. Five additional case reports have described: two patients with recurrent VTE while on therapeutic warfarin successfully treated with rivaroxaban and edoxaban, respectively; one patient successfully treated with dabigatran after developing warfarin-related hepatotoxicity, and two patients treated with rivaroxaban (1 had recurrent VTE on rivaroxaban; the other discontinued drug after one week and subsequently developed intra-cardiac thrombosis). A small, open-label randomized trial compared dalteparin to rivaroxaban in 16 NS patients with VTE and AT deficiency. The primary outcome was >90% resolution of thrombus at 4 weeks.57 Outcomes were similar in both groups suggesting similar rivaroxaban efficacy to LMWH. We recently reported an MN case with recurrent VTE on full-dose apixaban, but with sub-optimal peak drug levels.58 While DOAC safety and efficacy in NS patients awaits systematic prospective study, carefully selected DOACs may be a reasonable alternative for patients suffering recurrent VTE while on therapeutic warfarin (see Dosing Considerations).
Diabetic Nephropathy
Diabetes mellitus is not a strong independent VTE risk factor.59 However, diabetic nephropathy (DN) may confer increased VTE risk. The aforementioned Danish study demonstrated that DN was associated with higher adjusted VTE odds (OR 1.43 [95%CI 1.28–1.61]) compared to controls.2 Presently, no published data support specific anticoagulants for use in DN. However, a study of DOACs in type 2 diabetes demonstrated no differences in 2-hour pharmacokinetics or pharmacodynamics compared to non-diabetic controls for rivaroxaban, apixaban, or dabigatran.60 Data from the Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation (ARISTOTLE) trial demonstrated similar benefit for both diabetic and non-diabetic participants, suggesting diabetes itself did not influence efficacy61. However, DN patients with nephrotic-range proteinuria and/or hypoalbuminemia could exhibit altered DOAC metabolism. Cardiovascular events and cerebrovascular events are well known complications of diabetes. In patients with NS secondary to diabetes, arterial events were far more likely than VTE.62
Antiphospholipid Syndrome and Lupus Nephritis
Suggested primary prophylaxis (prior to first thrombosis) for patients with antiphospholipid antibodies (irrespective of SLE or LN status) is low-dose aspirin.63 Antiphospholipid syndrome (APS; antiphospholipid antibodies plus a thrombotic event) may be primary (without an underlying systemic autoimmune disorder) or may occur secondarily to a broader rheumatic disease.64 APS imparts a major predisposition to both arterial and venous thrombosis.65 Recommended therapy for patients with APS is long-term VKA with goal INR 2–3.63, 64, 66, 67 DOACs are generally not recommended for patients with APS. Most data regarding the management of APS coincident with kidney disease are in relation to lupus nephritis (LN). Systemic lupus erythematosus (SLE) patients are at increased VTE risk, but it is unclear if LN imparts additional VTE risk. One study examining renal outcomes in 66 patients with membranous LN (Class V LN) noted VTE in 15 (23%) patients over mean follow-up of 6.9 years.68 Most (93%) of these patients had secondary NS at the time of VTE. SLE patients may also develop antiphospholipid antibodies and secondary APS. The Rivaroxaban in Antiphospholipid Syndrome (RAPS) study evaluated rivaroxaban vs. warfarin in patients with APS (11% of whom had SLE).69 Over 6 months, no new thrombotic events were seen in either group. Triple positive APS (positive lupus anticoagulant plus both anti-cardiolipin and anti-β2 glycoprotein I antibodies) patients are at highest thrombotic risk and 28% of RAPS patients were triple positive. However, failure of rivaroxaban to prevent recurrent VTE has been reported in APS patients with and without triple positivity.70, 71 A systematic review of DOACs in APS identified 122 patients treated with DOACs, the majority (89%) of whom were treated with rivaroxaban (11% dabigatran; and one apixaban patient).72 Recurrent thrombotic events occurred in 19 patients and triple positivity was associated with 3.5-fold OR for recurrent thrombosis. The recently completed Trial on Rivaroxaban in AntiPhospholipid Syndrome (TRAPS) study, examined the non-inferiority of rivaroxaban vs. warfarin in recurrent VTE prevention in triple positive APS patients.73 TRAPS was concluded prematurely due to a higher incidence of the composite outcome (thromboembolic events, major bleeding, and vascular death) in the rivaroxaban group. A recently published non-inferiority study evaluated rivaroxaban versus VKA for secondary thromboprophylaxis in 190 patients with APS.74 Rivaroxaban did not meet the non-inferiority criteria and trended toward an increased risk of recurrent thrombotic events. Additional reports noted failure of dabigatran to prevent recurrent APS-associated VTE, not all of whom had triple positivity.75, 76 A current ongoing study is evaluating apixaban versus warfarin to prevent recurrent VTE in patients with APS ( NCT02295475).77 Considering these data, DOACs are not recommended for APS as first-line therapy. Some authors suggest that DOACs may be considered for patients who fail warfarin therapy, but we suggest that LMWH may be a better option.63 DOACs should be avoided in triple positive APS patients. Management of childhood APS is largely derived from adult data, thus these principles are generally applied to children.78, 79
ANCA Vasculitis
Anti-neutrophil cytoplasmic antibody (ANCA) vasculitis patients are known to be at high-risk for VTE. Data from cohort studies and clinical trials suggest that 8–16% of ANCA vasculitis patients develop VTE.80–83 In the Wegener’s Granulomatosis Etanercept Trial (WGET), 29 (16%) of 180 patients experienced VTE; 13 events occurred prior to enrollment.81 While this heightened VTE risk was initially reported primarily in granulomatosis with polyangiitis (GPA) ANCA patients, a study from the Netherlands reported the opposite, observing fewer events among PR3-positive patients.81, 84 Data from the European Vasculitis Study group suggested no difference in VTE risk between MPO+ vs. PR3+ patients.85 These data also identified higher serum creatinine and cutaneous or gastrointestinal involvement as VTE risk factors. In the Rituximab in ANCA-Associated Vasculitis (RAVE) trial, cardiac involvement, PR3 positivity, pulmonary hemorrhage, and urinary red blood cell (RBC) casts were identified as independent VTE risk factors.80 While most studies have focused on patients with GPA, microscopic polyangiitis or renal limited vasculitis, patients with eosinophilic granulomatosis with polyangiitis also have increased VTE risk.86 VTE have also been reported in children with ANCA vasculitis.87 The pathophysiology of ANCA vasculitis-associated hypercoagulopathy is likely multifactorial. Antibodies against plasminogen (inhibiting fibrinolysis) have been reported in both MPO+ and PR3+ patients.88, 89 Generation of tissue factor-bearing microparticles from inflamed vasculature may also contribute to the hypercoagulopathy.90, 91 Tissue factor may also be expressed within neutrophil extracellular traps (NETs) during ANCA vasculitis.91–93
Optimal management of thrombotic risk in ANCA vasculitis remains unknown. The majority of VTE occur during peak disease activity (even before diagnosis).80, 81, 84, 85 These patients are at risk for disease-associated pulmonary hemorrhage (which may also increase VTE risk); thus, risks and benefits of anticoagulation must be carefully balanced. Treatment of VTE has typically been warfarin or heparin in the acute phase. Well-established reversal protocols for these agents offer some security in the event of pulmonary hemorrhage. DOACs may be reasonable once disease is less active and pulmonary hemorrhage is less likely if continued therapy is indicated. Earlier use may be reasonable if DOAC reversal agents are readily available. Leukocytoclastic vasculitis hypersensitivity reactions have been reported in association with all DOACs and could confound assessment of vasculitis activity.94 This rare DOAC complication should not necessarily represent a contraindication. Optimal anticoagulant duration is unknown although it is reasonable to postulate VTE risk may be lower when vasculitis is quiescent. However, ANCA patients may exhibit hypercoagulability even in remission.95 A recent study demonstrated that peak microparticle-derived tissue factor activity (which did not necessarily correlate with disease activity) was associated with VTE.93 Further studies are thus needed to better define potential VTE-risk biomarkers in ANCA patients to guide anticoagulation prophylaxis and treatment.
Chronic Kidney Disease and End-Stage Kidney Disease
AF and VTE are both more common in patients with CKD or ESKD than in the general population. In a pooled analysis of five prospective cohorts including almost 600,000 European and United States participants, CKD (defined as estimated glomerular filtration rate (eGFR) <60 ml/1.73m2/min or albuminuria ≥30 mg/g creatinine) was associated with VTE (HR 1.54 [95% CI 1.15–2.06]).62 Even modest eGFR reductions were associated with elevated VTE risk. Similarly, AF occurs at higher rates and is associated with greater stroke risk in CKD and more so in ESKD patients.96, 97 Unfortunately, bleeding risks also increase making anticoagulation indications less clear.97, 98 Those with advanced CKD (eGFR <30 ml/1.73m2/min) deemed at high-risk for AF-related thromboembolic events likely benefit from warfarin anticoagulation.96 In contrast, some studies have demonstrated a lack of warfarin benefit in patients with ESKD on dialysis and potentially more bleeding events and stroke.99–101 Routine use of warfarin for AF in ESKD patients on dialysis is not recommended in the current KDIGO guidelines.102 Anticoagulation has been employed to maintain hemodialysis access patency but its utility for this purpose is unclear and generally not recommended.103
Data on DOACs in CKD are limited and largely derived from observational studies which may be confounded. The major randomized studies leading to DOAC approvals for non-valvular AF in the general population excluded patients with eGFR <25 ml/1.73m2/min.104 Despite this lack of trial data, DOAC use has become fairly routine in CKD care. For example, the Global Anticoagulant Registry in the FIELD-Atrial Fibrillation (GARFIELD-AF; a large registry of newly diagnosed AF cases) included 2,623 patients with moderate to severe CKD (stage 3–5), of whom 45.8% were placed on DOACs.105 Based upon these registry data and clinical trials, DOACs appear to be safe for patients with moderate CKD. A recent Cochrane review evaluated 12,545 patients with CKD across five large clinical trials who were randomized to a DOAC (apixaban, rivaroxaban, edoxaban or dabigatran).106 Compared to warfarin, DOACs demonstrated lower combined risk for stroke and systemic embolism (RR 0.81, 95% CI 0.65 to 1.00) and a trend toward less major bleeding (RR 0.79, 95% CI 0.59 to 1.04). The authors concluded that these agents were safe in CKD patients with the caveat that these data were primarily in patients with eGFR >30 ml/1.73m2/min. A recent retrospective, single center study included 6,412 patients with and without CKD (defined as eGFR <60 ml/1.73m2/min) and determined DOACs had similar benefit in stroke prevention with a small increase in bleeding.107 However, this study included few patients (N=8) with eGFR <30 ml/1.73m2/min. A recent study from Taiwan followed a prospective cohort of 3,771 patients with stage 4 or 5 CKD (about 25% on dialysis) with newly diagnosed AF.108 Notably only 21% of patients received anticoagulation - median eGFR in the group receiving DOACs was 25 ml/1.73m2/min compared to 17 ml/1.73m2/min in the warfarin and 16 ml/1.73m2/min in the non-anticoagulated group. DOAC and warfarin use were both associated with similar stroke risk to the non-anticoagulated group, but with higher bleeding event rates. A recent meta-analysis evaluated 45 trials of CKD or hemodialysis patients (8 trials) over a variety of indications.103 All of the hemodialysis trials only studied VKAs, the remaining studies excluded patients with severe CKD. In these analyses, DOACs had lower risk for stroke (RR 0.79, 95% CI 0.66 to 0.93) and hemorrhagic stroke (RR 0.48, 95% CI 0.30 to 0.76) than warfarin. The benefit of DOACs on recurrent VTE or VTE-related death were less clear but in the direction of benefit (RR 0.72, 95% CI 0.44 to 1.17) with lower bleeding risk.
DOAC use in ESKD has also become more common despite ESKD patients being excluded from the pivotal drug approval trials. The United States prescribing information for rivaroxaban and apixaban provide dose guidance for patients with ESKD. For non-valvular AF, rivaroxaban 15 mg daily is suggested and apixaban is suggested at the usual dose of 5 mg twice daily (unless dose reduction is indicated by age or weight). For VTE therapy and prophylaxis, no dose adjustment is suggested for ESKD patients. The suggested doses are reported to result in similar drug concentrations as observed in non-ESKD trials. However, these doses are based solely upon pharmacodynamic studies in ESKD patients without clinical outcome data.109 In retrospective claims analyses, rivaroxaban had an insignificantly decreased risk of ischemic stroke, but major bleeding was lower in the rivaroxaban group (HR 0.68, 95% CI 0.47–0.99) compared to warfarin.110 The majority of ESKD experience has been with apixaban. A large retrospective study used a claims data approach to match 25,523 ESKD patients with AF starting apixaban to those starting warfarin.111 No difference was noted in ischemic stroke risk but apixaban was associated with lower bleeding risk (HR 0.72, 95% CI 0.59–0.87). A sensitivity analysis of these data revealed that full dose apixaban (5 mg bid) was more favorable than reduced dose apixaban (2.5 mg bid) or warfarin in terms of stroke and mortality. Two other small retrospective studies demonstrated that apixaban has less bleeding-risk relative to warfarin in ESKD patients.112, 113 These latter studies also included patients treated for VTE; one noted no difference in VTE risk and the other noted an insignificantly lower VTE recurrence risk for DOACs compared to warfarin. While the benefit of any anticoagulant for AF in ESKD patients remains unclear, these observational data suggest DOACs may be safer than warfarin. Warfarin use is also a concern in ESKD patients (and in late-stage CKD) due to its potential contribution to vascular calcification and calciphylaxis. Thus, apixaban may prove to be a better option in ESKD patients at risk for these complications.114 Clinical trials are underway examining apixaban compared to warfarin in ESKD patients with AF ( NCT02933697).
Renal Transplantation
Renal transplant recipients also have elevated VTE risk.115 Unfortunately, little is known about DOACs in this setting. Acute changes in kidney function may occur due to acute rejection episodes, so it may be reasonable to avoid DOACs that are highly dependent on renal clearance due to the potential for wide pharmacodynamic fluctuations. However, the reversal agents may mitigate any risk associated with fluctuating drug clearance. Apixaban and rivaroxaban are reasonable choices if dosed appropriate to kidney function (Table 2). Drug interactions between calcineurin inhibitors (CNI) and DOACs do exist (see Dosing Considerations). Thus, immunosuppressant monitoring and/or DOAC dose adjustment are important considerations.116 Caution should be employed in this unique population due to possible polypharmacy with additional agents that may influence DOAC metabolism.
SPECIAL CONSIDERATIONS
Anticoagulant-Related Nephropathy
Anticoagulant-related nephropathy (ARN) was first described in patients on warfarin with acute kidney injury (AKI).117 Clinical findings included hematuria and AKI in patients with supratherapeutic INRs (>3) without other known AKI causes. Kidney biopsies demonstrated dysmorphic RBCs in Bowman’s space, RBC casts in dilated distal tubules, and tubular hemosiderin deposition. Hypothesized injury mechanisms are nephron obstruction by RBC casts or tubular oxidative stress from RBC-derived iron.118 While not initially widely accepted, the association between anticoagulation and AKI has been confirmed in several additional studies and may be more common that initially thought.119–121 For example, in one retrospective non-biopsy study, 20.5% of patients treated with warfarin with a first INR >3 developed AKI within 1 week; those with AKI had a 65% increased risk of mortality within 1 year.122 Risk factors for ARN include CKD, older age, diabetes mellitus, hypertension, and cardiovascular disease.122 Nonetheless, these largely retrospective studies may be subject to bias, including potential reverse causality. Accelerated progression of CKD has also been attributed to recurrent episodes of ARN. DOACs have also been associated with ARN, although with lower risk than warfarin. An early meta-analysis of ten RCTs demonstrated similar AKI risk in patients treated with dabigatran, apixaban, or rivaroxaban compared to warfarin.123 In contrast, a large retrospective study from Taiwan demonstrated a lower risk of AKI with apixaban, dabigatran, and rivaroxaban compared to warfarin.124 Retrospective analysis of a United States cohort similarly demonstrated lower AKI risk and lower risk of ≥30% decline in eGFR with DOAC treatment compared to warfarin.125 Moreover, post-hoc analyses of the Randomized Evaluation of Long Term Anticoagulant Therapy (RE-LY) and Rivaroxaban Once-daily Compared with Vitamin K Antagonism for Prevention of Embolism Trial in Atrial Fibrillation (ROCKET AF) trials demonstrated slower CKD progression with dabigatran or rivaroxaban, respectively, compared to warfarin.126, 127 Warfarin is also known to contribute to vascular calcification, which may indirectly contribute to CKD progression.128
Dosing Considerations
Kidney Function
All DOACs are renally excreted, varying between 11–80% (Table 1). Dose adjustments are required for reduced CrCl and based upon anticoagulant indication and regulatory agency (i.e. FDA vs. EMA; Table 2).45 A prescription data analysis showed that 28% of CKD patients prescribed DOACs did not receive recommended dose reductions.107 Notably, all pivotal, phase 3 DOAC trials used CrCl determined by Cockcroft-Gault to estimate kidney function rather than eGFR and excluded patients with CrCl <25–30 ml/min. Both Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) and Modification of Diet in Renal Disease (MDRD) eGFR equations produce higher estimates of renal function compared to CrCl.129 A study of 185 consecutive patients with AF found that using the CKD-EPI formula to determine DOAC dosing would cause 48% of patients with CrCl <30 ml/min and 46% with CrCl 30–49.9 ml/min to receive treatment inconsistent with guidelines.129 A study utilizing National Health and Nutrition Examination Survey (NHANES) data similarly demonstrated that substitution of MDRD eGFR for estimated CrCl can lead to higher dosing of DOACs and potentially increased bleeding rates.130 Thus, DOAC dosing should be determined using Cockcroft-Gault CrCl for CKD patients.
Nephrotic Syndrome
Anticoagulant dosing in NS patients is complex due to altered pharmacokinetic properties associated with hypoalbuminemia and altered protein binding (Table 1), increased volume of distribution, and fluctuations in CrCl. A pharmacokinetic study of warfarin, which is highly protein bound, demonstrated threefold higher plasma clearance and twofold shorter half-life in NS patients vs. controls, frequent INR monitoring is thus recommended for NS patients.131 Experience with DOACs in NS is limited primarily to case reports demonstrating effective primary prophylaxis with apixaban57 and effective treatment of VTE with rivaroxaban, edoxaban, and dabigatran.132–134 However, recurrent VTE in a patient with MN while on therapeutic dosing of apixaban has also been described.58 The authors reported a lower peak apixaban concentration and hypothesized higher unbound drug fraction led to more rapid excretion, shorter half-life, and increased VTE risk. Among the DOACs, plasma protein binding ranges from 35–95%; NS-specific studies are thus needed for each drug to establish dosing recommendations in this population.
Drug/Drug Interactions
Polypharmacy is prevalent in CKD patients and increases risks for adverse drug interactions. Warfarin is metabolized by CYP2C9; thus CYP2C9 inhibitors (e.g. amiodarone, fluconazole, fluvastatin, isoniazid, sertraline) may increase anticoagulant effects whereas CYP2C9 inducers (e.g. rifampin) reduce them.135 There are also important potential drug interactions relevant to DOACs.136 Dabigatran is a substrate for P-glycoprotein, thus treatment with P-glycoprotein inducers (e.g. rifampin, phenobarbital, phenytoin) may decrease anticoagulant effects and increase risk of treatment failure. Co-treatment with P-glycoprotein inhibitors (e.g. digoxin, cyclosporine, verapamil, diltiazem, amiodarone) may increase anticoagulant effects and bleeding risk. Particular care should be taken in the CKD setting where the combination of reduced renal clearance and co-administration of P-glycoprotein inhibitors may increase bleeding risk.137 Factor Xa DOACs also depend on the P-glycoprotein pathway for metabolism, and interactions with inducers and inhibitors have been described. Moreover, factor Xa DOACs are metabolized by cytochrome P450 and drug effectiveness may be affected by co-administration with CYP3A4 inhibitors (e.g. fluconazole, ketoconazole, itraconazole, vorconazole) or CYP3A4 inducers (e.g. rifampin, phenytoin). These drug interactions increase bleeding risk or treatment failure, respectively. An administrative database review of 91,330 patients with AF in Taiwan treated with DOACs found an increased risk of major bleeding in those co-prescribed amiodarone, fluconazole, rifampin, and phenytoin.138 Close consultation with an experienced pharmacist may be helpful in minimizing adverse events from drug interactions.
Childhood Kidney Disease
Children with CKD have higher VTE risk than the general pediatric population.3 Risk factors include NS, infection, recent trauma/surgery, central venous catheters, and ESKD/dialysis.3 LMWH has been standard therapy for children with VTE for over 20 years, with dose adjustments for CrCl <30 ml/min, similar to adults.139 DOACs are potentially attractive agents in this population due to oral administration and no need for timed lab monitoring. However, none are currently approved for pediatric use. However, phase 2 pediatric trials of rivaroxaban and dabigatran were recently published and phase 3 trials ( NCT02234843 and NCT01895777, respectively) are nearing completion.140, 141 Thus, these agents may soon be available for pediatric use. Anticoagulant dosing in children is complex due to age related hemostasis-system maturation as well as pharmacokinetic differences compared to adults.142 Only dabigatran has published pharmacokinetic data in children showing similarities to adults with the exception of longer clotting times in children <1 year of age.143 Pediatric pharmacokinetic data for the remainder of the DOACs is limited to in vitro experiments144 and case reports that suggest possible differences compared to adults.145 Similar to the adult studies, children with CrCl <30–50 ml/min are excluded from the aforementioned studies, making it unlikely that safety and efficacy data will be generated for childhood CKD in the near term.
CONCLUSIONS
DOACs are increasingly utilized in a variety of kidney disease settings, despite the lack of controlled trial data. The field would benefit from carefully designed trials that consider renal function, intravascular volume, plasma protein-drug binding, and interactions with commonly employed medications, in each type of kidney disease where anticoagulation is frequently indicated. While the early forms of data described in this review suggest that DOACs hold promise for improved efficacy and safety in the setting of kidney disease, this can only be confirmed through meticulous prospective study.
ACKNOWLEDGEMENTS
We are indebted to Ms. Lisa Feurer for her graphical design expertise (Figure).
FUNDING
This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health (Award Numbers K08DK103982 and R03DK118315 to BAK; Award Numbers U54DK083912 and P01DK058335 to VKD). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
DISCLOSURES
MNR is a site primary investigator for clinical trials for Retrophin, Advicenne, Reata, Genentech and has received research funding from Goldfinch Bio, Novartis, NIDDK, and Department of Defense unrelated to direct oral anticoagulants. VKD has received consultant fees from Novartis and Retrophin and honoraria from RTI International and UpToDate unrelated to direct oral anticoagulants. VKD is, or has been, a site primary investigator in multiple clinical trials for Mallinckrodt, Gilead, Bristol-Myers Squibb, InflaRx, Chemocentryx, Otsuka and Retrophin. BAK has received research funding from Novo Nordisk A/S and CSL Behring, unrelated to direct oral anticoagulants. BAK is, or has been, a site primary investigator in multiple clinical trials for Bayer Healthcare, CSL Behring, Novo Nordisk A/S, and Bioveritiv and has received consultant fees or honoraria from Bayer Healthcare, Novo Nordisk A/S, and CSL Behring.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Shroff GR, Chang TI. Risk Stratification and Treatment of Coronary Disease in Chronic Kidney Disease and End-Stage Kidney Disease. Semin Nephrol 2018; 38: 582–599. [DOI] [PubMed] [Google Scholar]
- 2.Christiansen CF, Schmidt M, Lamberg AL, et al. Kidney disease and risk of venous thromboembolism: a nationwide population-based case-control study. J Thromb Haemost 2014; 12: 1449–1454. [DOI] [PubMed] [Google Scholar]
- 3.Kerlin BA, Smoyer WE, Tsai J, et al. Healthcare burden of venous thromboembolism in childhood chronic renal diseases. Pediatr Nephrol 2015; 30: 829–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pavord S, Myers B. Bleeding and thrombotic complications of kidney disease. Blood Rev 2011; 25: 271–278. [DOI] [PubMed] [Google Scholar]
- 5.Ghanta M, Kozicky M, Jim B. Pathophysiologic and treatment strategies for cardiovascular disease in end-stage renal disease and kidney transplantations. Cardiol Rev 2015; 23: 109–118. [DOI] [PubMed] [Google Scholar]
- 6.Kahn MR, Robbins MJ, Kim MC, et al. Management of cardiovascular disease in patients with kidney disease. Nat Rev Cardiol 2013; 10: 261–273. [DOI] [PubMed] [Google Scholar]
- 7.Mathew RO, Bangalore S, Lavelle MP, et al. Diagnosis and management of atherosclerotic cardiovascular disease in chronic kidney disease: a review. Kidney Int 2017; 91: 797–807. [DOI] [PubMed] [Google Scholar]
- 8.Mavrakanas TA, Charytan DM. Cardiovascular complications in chronic dialysis patients. Curr Opin Nephrol Hypertens 2016; 25: 536–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shoji T, Abe T, Matsuo H, et al. Chronic kidney disease, dyslipidemia, and atherosclerosis. J Atheroscler Thromb 2012; 19: 299–315. [DOI] [PubMed] [Google Scholar]
- 10.Stoumpos S, Jardine AG, Mark PB. Cardiovascular morbidity and mortality after kidney transplantation. Transpl Int 2015; 28: 10–21. [DOI] [PubMed] [Google Scholar]
- 11.Eikelboom JW, Connolly SJ, Bosch J, et al. Rivaroxaban with or without Aspirin in Stable Cardiovascular Disease. N Engl J Med 2017; 377: 1319–1330. [DOI] [PubMed] [Google Scholar]
- 12.Mega JL, Braunwald E, Wiviott SD, et al. Rivaroxaban in patients with a recent acute coronary syndrome. N Engl J Med 2012; 366: 9–19. [DOI] [PubMed] [Google Scholar]
- 13.Ibrahim H, Rao SV. Oral antiplatelet drugs in patients with chronic kidney disease (CKD): a review. J Thromb Thrombolysis 2017; 43: 519–527. [DOI] [PubMed] [Google Scholar]
- 14.Jain N, Hedayati SS, Sarode R, et al. Antiplatelet therapy in the management of cardiovascular disease in patients with CKD: what is the evidence? Clin J Am Soc Nephrol 2013; 8: 665–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Migliori M, Cantaluppi V, Scatena A, et al. Antiplatelet agents in hemodialysis. J Nephrol 2017; 30: 373–383. [DOI] [PubMed] [Google Scholar]
- 16.Palmer SC, Di Micco L, Razavian M, et al. Antiplatelet agents for chronic kidney disease. Cochrane Database Syst Rev 2013: CD008834. [DOI] [PubMed] [Google Scholar]
- 17.Bansal N, Hsu CY, Go AS. Intersection of cardiovascular disease and kidney disease: atrial fibrillation. Curr Opin Nephrol Hypertens 2014; 23: 275–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alonso A, Lopez FL, Matsushita K, et al. Chronic kidney disease is associated with the incidence of atrial fibrillation: the Atherosclerosis Risk in Communities (ARIC) study. Circulation 2011; 123: 2946–2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ananthapanyasut W, Napan S, Rudolph EH, et al. Prevalence of atrial fibrillation and its predictors in nondialysis patients with chronic kidney disease. Clin J Am Soc Nephrol 2010; 5: 173–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baber U, Howard VJ, Halperin JL, et al. Association of chronic kidney disease with atrial fibrillation among adults in the United States: REasons for Geographic and Racial Differences in Stroke (REGARDS) Study. Circ Arrhythm Electrophysiol 2011; 4: 26–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Horio T, Iwashima Y, Kamide K, et al. Chronic kidney disease as an independent risk factor for new-onset atrial fibrillation in hypertensive patients. J Hypertens 2010; 28: 1738–1744. [DOI] [PubMed] [Google Scholar]
- 22.Soliman EZ, Prineas RJ, Go AS, et al. Chronic kidney disease and prevalent atrial fibrillation: the Chronic Renal Insufficiency Cohort (CRIC). Am Heart J 2010; 159: 1102–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wetmore JB, Mahnken JD, Rigler SK, et al. The prevalence of and factors associated with chronic atrial fibrillation in Medicare/Medicaid-eligible dialysis patients. Kidney Int 2012; 81: 469–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Winkelmayer WC, Patrick AR, Liu J, et al. The increasing prevalence of atrial fibrillation among hemodialysis patients. J Am Soc Nephrol 2011; 22: 349–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boulet SL, Grosse SD, Thornburg CD, et al. Trends in venous thromboembolism-related hospitalizations, 1994–2009. Pediatrics 2012; 130: e812–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hirsh J, Warkentin TE, Raschke R, et al. Heparin and low-molecular-weight heparin: mechanisms of action, pharmacokinetics, dosing considerations, monitoring, efficacy, and safety. Chest 1998; 114: 489S–510S. [DOI] [PubMed] [Google Scholar]
- 27.Manzoor BS, Cheng WH, Lee JC, et al. Quality of Pharmacist-Managed Anticoagulation Therapy in Long-Term Ambulatory Settings: A Systematic Review. Ann Pharmacother 2017; 51: 1122–1137. [DOI] [PubMed] [Google Scholar]
- 28.Kleinow ME, Garwood CL, Clemente JL, et al. Effect of chronic kidney disease on warfarin management in a pharmacist-managed anticoagulation clinic. J Manag Care Pharm 2011; 17: 523–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kerlin BA. Current and future management of pediatric venous thromboembolism. American journal of hematology 2012; 87 Suppl 1: S68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kathiresan S, Shiomura J, Jang IK. Argatroban. J Thromb Thrombolysis 2002; 13: 41–47. [DOI] [PubMed] [Google Scholar]
- 31.Warkentin TE, Greinacher A, Koster A. Bivalirudin. Thromb Haemost 2008; 99: 830–839. [DOI] [PubMed] [Google Scholar]
- 32.Graetz TJ, Tellor BR, Smith JR, et al. Desirudin: a review of the pharmacology and clinical application for the prevention of deep vein thrombosis. Expert Rev Cardiovasc Ther 2011; 9: 1101–1109. [DOI] [PubMed] [Google Scholar]
- 33.Barnes GD, Ageno W, Ansell J, et al. Recommendation on the nomenclature for oral anticoagulants: communication from the SSC of the ISTH. J Thromb Haemost 2015; 13: 1154–1156. [DOI] [PubMed] [Google Scholar]
- 34.Katz DF, Maddox TM, Turakhia M, et al. Contemporary Trends in Oral Anticoagulant Prescription in Atrial Fibrillation Patients at Low to Moderate Risk of Stroke After Guideline-Recommended Change in Use of the CHADS2 to the CHA2DS2-VASc Score for Thromboembolic Risk Assessment: Analysis From the National Cardiovascular Data Registry’s Outpatient Practice Innovation and Clinical Excellence Atrial Fibrillation Registry. Circ Cardiovasc Qual Outcomes 2017; 10. [DOI] [PubMed] [Google Scholar]
- 35.Vinogradova Y, Coupland C, Hill T, et al. Risks and benefits of direct oral anticoagulants versus warfarin in a real world setting: cohort study in primary care. BMJ 2018; 362: k2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Laizure SC, Parker RB, Herring VL, et al. Identification of carboxylesterase-dependent dabigatran etexilate hydrolysis. Drug metabolism and disposition: the biological fate of chemicals 2014; 42: 201–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blech S, Ebner T, Ludwig-Schwellinger E, et al. The metabolism and disposition of the oral direct thrombin inhibitor, dabigatran, in humans. Drug metabolism and disposition: the biological fate of chemicals 2008; 36: 386–399. [DOI] [PubMed] [Google Scholar]
- 38.Huisman MV, Klok FA. Pharmacological properties of betrixaban. Eur Heart J Suppl 2018; 20: E12–E15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lu G, Pine P, Leeds JM, et al. Andexanet alfa effectively reverses edoxaban anticoagulation effects and associated bleeding in a rabbit acute hemorrhage model. PLoS One 2018; 13: e0195122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Milling TJ, Jr, Ziebell CM. A review of reversal of oral anticoagulants, old and new, in major bleeding and the need for urgent surgery. Trends Cardiovasc Med 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yeh CH, Fredenburgh JC, Weitz JI. Oral direct factor Xa inhibitors. Circ Res 2012; 111: 1069–1078. [DOI] [PubMed] [Google Scholar]
- 42.Pollack CV, Jr., Reilly PA, van Ryn J, et al. Idarucizumab for Dabigatran Reversal - Full Cohort Analysis. N Engl J Med 2017; 377: 431–441. [DOI] [PubMed] [Google Scholar]
- 43.Connolly SJ, Milling TJ, Jr, Eikelboom JW, et al. Andexanet Alfa for Acute Major Bleeding Associated with Factor Xa Inhibitors. N Engl J Med 2016; 375: 1131–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dobesh PP, Bhatt SH, Trujillo TC, et al. Antidotes for reversal of direct oral anticoagulants. Pharmacol Ther 2019: 107405. [DOI] [PubMed] [Google Scholar]
- 45.Parker K, Thachil J. The use of direct oral anticoagulants in chronic kidney disease. Br J Haematol 2018; 183: 170–184. [DOI] [PubMed] [Google Scholar]
- 46.Blainey JD, Hardwicke J, Whitfield AG. The nephrotic syndrome associated with thrombosis of the renal veins. Lancet 1954; 267: 1208–1211. [DOI] [PubMed] [Google Scholar]
- 47.Kerlin BA, Ayoob R, Smoyer WE. Epidemiology and pathophysiology of nephrotic syndrome-associated thromboembolic disease. Clin J Am Soc Nephrol 2012; 7: 513–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Loscalzo J Venous thrombosis in the nephrotic syndrome. N Engl J Med 2013; 368: 956–958. [DOI] [PubMed] [Google Scholar]
- 49.Kerlin BA, Blatt NB, Fuh B, et al. Epidemiology and risk factors for thromboembolic complications of childhood nephrotic syndrome: a Midwest Pediatric Nephrology Consortium (MWPNC) study. J Pediatr 2009; 155: 105–110, 110 e101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Barbour SJ, Greenwald A, Djurdjev O, et al. Disease-specific risk of venous thromboembolic events is increased in idiopathic glomerulonephritis. Kidney Int 2012; 81: 190–195. [DOI] [PubMed] [Google Scholar]
- 51.Lionaki S, Derebail VK, Hogan SL, et al. Venous thromboembolism in patients with membranous nephropathy. Clin J Am Soc Nephrol 2012; 7: 43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.KDIGO Clinical Practice Guideline for Glomerulonephritis. Kidney Int Suppl 2012; 2: 139–274. [Google Scholar]
- 53.Lee T, Biddle AK, Lionaki S, et al. Personalized prophylactic anticoagulation decision analysis in patients with membranous nephropathy. Kidney Int 2014; 85: 1412–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rostoker G, Durand-Zaleski I, Petit-Phar M, et al. Prevention of thrombotic complications of the nephrotic syndrome by the low-molecular-weight heparin enoxaparin. Nephron 1995; 69: 20–28. [DOI] [PubMed] [Google Scholar]
- 55.Kelddal S, Nykjaer KM, Gregersen JW, et al. Prophylactic anticoagulation in nephrotic syndrome prevents thromboembolic complications. BMC Nephrol 2019; 20: 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sexton DJ, de Freitas DG, Little MA, et al. Direct-Acting Oral Anticoagulants as Prophylaxis Against Thromboembolism in the Nephrotic Syndrome. Kidney Int Rep 2018; 3: 784–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang L, Zhang H, Zhang J, et al. Rivaroxaban for the treatment of venous thromboembolism in patients with nephrotic syndrome and low AT-III: A pilot study. Exp Ther Med 2018; 15: 739–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Reynolds ML, Nachman PH, Mooberry MJ, et al. Recurrent venous thromboembolism in primary membranous nephropathy despite direct Xa inhibitor therapy. J Nephrol 2019; 32: 669–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Heit JA, Leibson CL, Ashrani AA, et al. Is diabetes mellitus an independent risk factor for venous thromboembolism?: a population-based case-control study. Arterioscler Thromb Vasc Biol 2009; 29: 1399–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Samos M, Bolek T, Stanciakova L, et al. Does type 2 diabetes affect the on-treatment levels of direct oral anticoagulants in patients with atrial fibrillation? Diabetes Res Clin Pract 2018; 135: 172–177. [DOI] [PubMed] [Google Scholar]
- 61.Ezekowitz JA, Lewis BS, Lopes RD, et al. Clinical outcomes of patients with diabetes and atrial fibrillation treated with apixaban: results from the ARISTOTLE trial. Eur Heart J Cardiovasc Pharmacother 2015; 1: 86–94. [DOI] [PubMed] [Google Scholar]
- 62.Mahmoodi BK, ten Kate MK, Waanders F, et al. High absolute risks and predictors of venous and arterial thromboembolic events in patients with nephrotic syndrome: results from a large retrospective cohort study. Circulation 2008; 117: 224–230. [DOI] [PubMed] [Google Scholar]
- 63.Tektonidou MG, Andreoli L, Limper M, et al. EULAR recommendations for the management of antiphospholipid syndrome in adults. Ann Rheum Dis 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost 2006; 4: 295–306. [DOI] [PubMed] [Google Scholar]
- 65.Cervera R Antiphospholipid syndrome. Thromb Res 2017; 151 Suppl 1: S43–S47. [DOI] [PubMed] [Google Scholar]
- 66.Lim W Antiphospholipid syndrome. Hematology Am Soc Hematol Educ Program 2013; 2013: 675–680. [DOI] [PubMed] [Google Scholar]
- 67.Ruiz-Irastorza G, Cuadrado MJ, Ruiz-Arruza I, et al. Evidence-based recommendations for the prevention and long-term management of thrombosis in antiphospholipid antibody-positive patients: report of a task force at the 13th International Congress on antiphospholipid antibodies. Lupus 2011; 20: 206–218. [DOI] [PubMed] [Google Scholar]
- 68.Mercadal L, Montcel ST, Nochy D, et al. Factors affecting outcome and prognosis in membranous lupus nephropathy. Nephrol Dial Transplant 2002; 17: 1771–1778. [DOI] [PubMed] [Google Scholar]
- 69.Cohen H, Hunt BJ, Efthymiou M, et al. Rivaroxaban versus warfarin to treat patients with thrombotic antiphospholipid syndrome, with or without systemic lupus erythematosus (RAPS): a randomised, controlled, open-label, phase 2/3, non-inferiority trial. Lancet Haematol 2016; 3: e426–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dufrost V, Risse J, Kirchner S, et al. Failure of rivaroxaban to prevent thrombosis in four patients with anti-phospholipid syndrome. Rheumatology (Oxford) 2017; 56: 1433–1434. [DOI] [PubMed] [Google Scholar]
- 71.Signorelli F, Nogueira F, Domingues V, et al. Thrombotic events in patients with antiphospholipid syndrome treated with rivaroxaban: a series of eight cases. Clin Rheumatol 2016; 35: 801–805. [DOI] [PubMed] [Google Scholar]
- 72.Dufrost V, Risse J, Zuily S, et al. Direct Oral Anticoagulants Use in Antiphospholipid Syndrome: Are These Drugs an Effective and Safe Alternative to Warfarin? A Systematic Review of the Literature. Curr Rheumatol Rep 2016; 18: 74. [DOI] [PubMed] [Google Scholar]
- 73.Pengo V, Denas G, Zoppellaro G, et al. Rivaroxaban vs warfarin in high-risk patients with antiphospholipid syndrome. Blood 2018; 132: 1365–1371. [DOI] [PubMed] [Google Scholar]
- 74.Ordi-Ros J, Saez-Comet L, Perez-Conesa M, et al. Rivaroxaban Versus Vitamin K Antagonist in Antiphospholipid Syndrome: A Randomized Noninferiority Trial. Ann Intern Med 2019. [DOI] [PubMed] [Google Scholar]
- 75.Schaefer JK, McBane RD, Black DF, et al. Failure of dabigatran and rivaroxaban to prevent thromboembolism in antiphospholipid syndrome: a case series of three patients. Thromb Haemost 2014; 112: 947–950. [DOI] [PubMed] [Google Scholar]
- 76.Johnsen S, Lauvsnes MB, Omdal R. Treatment failure of direct oral anticoagulants in anti-phospholipid syndrome. Scand J Rheumatol 2018; 47: 427–428. [DOI] [PubMed] [Google Scholar]
- 77.Woller SC, Stevens SM, Kaplan DA, et al. Apixaban for the Secondary Prevention of Thrombosis Among Patients With Antiphospholipid Syndrome: Study Rationale and Design (ASTRO-APS). Clin Appl Thromb Hemost 2016; 22: 239–247. [DOI] [PubMed] [Google Scholar]
- 78.Tarango C, Palumbo JS. Antiphospholipid syndrome in pediatric patients. Curr Opin Hematol 2019; 26: 366–371. [DOI] [PubMed] [Google Scholar]
- 79.Monagle P, Chan AKC, Goldenberg NA, et al. Antithrombotic therapy in neonates and children: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141: e737S–e801S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kronbichler A, Leierer J, Shin JI, et al. The Association of Pulmonary Haemorrhage, Positive PR3-ANCA and Urinary Red Blood Cell Casts with Venous Thromboembolism in ANCA-Associated Vasculitis. Arthritis Rheumatol 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Merkel PA, Lo GH, Holbrook JT, et al. Brief communication: high incidence of venous thrombotic events among patients with Wegener granulomatosis: the Wegener’s Clinical Occurrence of Thrombosis (WeCLOT) Study. Ann Intern Med 2005; 142: 620–626. [DOI] [PubMed] [Google Scholar]
- 82.Faurschou M, Obel N, Baslund B. High risk of pulmonary embolism and deep venous thrombosis but not of stroke in granulomatosis with polyangiitis (Wegener’s). Arthritis Care Res (Hoboken) 2014; 66: 1910–1914. [DOI] [PubMed] [Google Scholar]
- 83.Berti A, Matteson EL, Crowson CS, et al. Risk of Cardiovascular Disease and Venous Thromboembolism Among Patients With Incident ANCA-Associated Vasculitis: A 20-Year Population-Based Cohort Study. Mayo Clin Proc 2018; 93: 597–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Weidner S, Hafezi-Rachti S, Rupprecht HD. Thromboembolic events as a complication of antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheum 2006; 55: 146–149. [DOI] [PubMed] [Google Scholar]
- 85.Kronbichler A, Leierer J, Leierer G, et al. Clinical associations with venous thromboembolism in anti-neutrophil cytoplasm antibody-associated vasculitides. Rheumatology (Oxford) 2017; 56: 704–708. [DOI] [PubMed] [Google Scholar]
- 86.Allenbach Y, Seror R, Pagnoux C, et al. High frequency of venous thromboembolic events in Churg-Strauss syndrome, Wegener’s granulomatosis and microscopic polyangiitis but not polyarteritis nodosa: a systematic retrospective study on 1130 patients. Ann Rheum Dis 2009; 68: 564–567. [DOI] [PubMed] [Google Scholar]
- 87.Tseng ST, Tseng MH, Huang JL. Concurrent pulmonary hemorrhage and deep vein thrombosis in a child with ANCA-associated vasculitis: case report and review of literature. Pediatr Rheumatol Online J 2015; 13: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bautz DJ, Preston GA, Lionaki S, et al. Antibodies with dual reactivity to plasminogen and complementary PR3 in PR3-ANCA vasculitis. J Am Soc Nephrol 2008; 19: 2421–2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Berden AE, Nolan SL, Morris HL, et al. Anti-plasminogen antibodies compromise fibrinolysis and associate with renal histology in ANCA-associated vasculitis. J Am Soc Nephrol 2010; 21: 2169–2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hong Y, Eleftheriou D, Hussain AA, et al. Anti-neutrophil cytoplasmic antibodies stimulate release of neutrophil microparticles. J Am Soc Nephrol 2012; 23: 49–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kambas K, Chrysanthopoulou A, Vassilopoulos D, et al. Tissue factor expression in neutrophil extracellular traps and neutrophil derived microparticles in antineutrophil cytoplasmic antibody associated vasculitis may promote thromboinflammation and the thrombophilic state associated with the disease. Ann Rheum Dis 2014; 73: 1854–1863. [DOI] [PubMed] [Google Scholar]
- 92.Huang YM, Wang H, Wang C, et al. Promotion of hypercoagulability in antineutrophil cytoplasmic antibody-associated vasculitis by C5a-induced tissue factor-expressing microparticles and neutrophil extracellular traps. Arthritis Rheumatol 2015; 67: 2780–2790. [DOI] [PubMed] [Google Scholar]
- 93.Mendoza CE, Brant EJ, McDermott ML, et al. Elevated Microparticle Tissue Factor Activity Differentiates Patients with Venous Thromboembolism in Anti-neutrophil Cytoplasmic Autoantibody Vasculitis. Kidney International Reports. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Carli G, Farsi A, Chiarini F, et al. Hypersensitivity reactions to non-vitamin K oral anticoagulants - a review of literature and diagnostic work-up proposal. Eur Ann Allergy Clin Immunol 2019; 51: 7–14. [DOI] [PubMed] [Google Scholar]
- 95.Hilhorst M, Winckers K, Wilde B, et al. Patients with antineutrophil cytoplasmic antibodies associated vasculitis in remission are hypercoagulable. J Rheumatol 2013; 40: 2042–2046. [DOI] [PubMed] [Google Scholar]
- 96.Bonde AN, Lip GY, Kamper AL, et al. Net clinical benefit of antithrombotic therapy in patients with atrial fibrillation and chronic kidney disease: a nationwide observational cohort study. J Am Coll Cardiol 2014; 64: 2471–2482. [DOI] [PubMed] [Google Scholar]
- 97.Clase CM, Holden RM, Sood MM, et al. Should patients with advanced chronic kidney disease and atrial fibrillation receive chronic anticoagulation? Nephrol Dial Transplant 2012; 27: 3719–3724. [DOI] [PubMed] [Google Scholar]
- 98.Olesen JB, Lip GY, Kamper AL, et al. Stroke and bleeding in atrial fibrillation with chronic kidney disease. N Engl J Med 2012; 367: 625–635. [DOI] [PubMed] [Google Scholar]
- 99.Yoon CY, Noh J, Jhee JH, et al. Warfarin Use in Patients With Atrial Fibrillation Undergoing Hemodialysis: A Nationwide Population-Based Study. Stroke 2017; 48: 2472–2479. [DOI] [PubMed] [Google Scholar]
- 100.Chan KE, Lazarus JM, Thadhani R, et al. Warfarin use associates with increased risk for stroke in hemodialysis patients with atrial fibrillation. J Am Soc Nephrol 2009; 20: 2223–2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shah M, Avgil Tsadok M, Jackevicius CA, et al. Warfarin use and the risk for stroke and bleeding in patients with atrial fibrillation undergoing dialysis. Circulation 2014; 129: 1196–1203. [DOI] [PubMed] [Google Scholar]
- 102.Wanner C, Herzog CA, Turakhia MP, et al. Chronic kidney disease and arrhythmias: highlights from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int 2018; 94: 231–234. [DOI] [PubMed] [Google Scholar]
- 103.Ha JT, Neuen BL, Cheng LP, et al. Benefits and Harms of Oral Anticoagulant Therapy in Chronic Kidney Disease: A Systematic Review and Meta-analysis. Ann Intern Med 2019. [DOI] [PubMed] [Google Scholar]
- 104.Chan KE, Giugliano RP, Patel MR, et al. Nonvitamin K Anticoagulant Agents in Patients With Advanced Chronic Kidney Disease or on Dialysis With AF. J Am Coll Cardiol 2016; 67: 2888–2899. [DOI] [PubMed] [Google Scholar]
- 105.Haas S, Camm AJ, Bassand JP, et al. Predictors of NOAC versus VKA use for stroke prevention in patients with newly diagnosed atrial fibrillation: Results from GARFIELD-AF. Am Heart J 2019; 213: 35–46. [DOI] [PubMed] [Google Scholar]
- 106.Kimachi M, Furukawa TA, Kimachi K, et al. Direct oral anticoagulants versus warfarin for preventing stroke and systemic embolic events among atrial fibrillation patients with chronic kidney disease. Cochrane Database Syst Rev 2017; 11: CD011373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shin JI, Secora A, Alexander GC, et al. Risks and Benefits of Direct Oral Anticoagulants across the Spectrum of GFR among Incident and Prevalent Patients with Atrial Fibrillation. Clin J Am Soc Nephrol 2018; 13: 1144–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chang SH, Wu CV, Yeh YH, et al. Efficacy and Safety of Oral Anticoagulants in Patients with Atrial Fibrillation and Stages 4 or 5 Chronic Kidney Disease. Am J Med 2019. [DOI] [PubMed] [Google Scholar]
- 109.Burlacu A, Genovesi S, Ortiz A, et al. Pros and cons of antithrombotic therapy in end-stage kidney disease: a 2019 update. Nephrol Dial Transplant 2019; 34: 923–933. [DOI] [PubMed] [Google Scholar]
- 110.Coleman CI, Kreutz R, Sood NA, et al. Rivaroxaban Versus Warfarin in Patients With Nonvalvular Atrial Fibrillation and Severe Kidney Disease or Undergoing Hemodialysis. Am J Med 2019. [DOI] [PubMed] [Google Scholar]
- 111.Siontis KC, Zhang X, Eckard A, et al. Outcomes Associated With Apixaban Use in Patients With End-Stage Kidney Disease and Atrial Fibrillation in the United States. Circulation 2018; 138: 1519–1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Schafer JH, Casey AL, Dupre KA, et al. Safety and Efficacy of Apixaban Versus Warfarin in Patients With Advanced Chronic Kidney Disease. Ann Pharmacother 2018; 52: 1078–1084. [DOI] [PubMed] [Google Scholar]
- 113.Reed D, Palkimas S, Hockman R, et al. Safety and effectiveness of apixaban compared to warfarin in dialysis patients. Res Pract Thromb Haemost 2018; 2: 291–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Garza-Mayers AC, Shah R, Sykes DB, et al. The Successful Use of Apixaban in Dialysis Patients with Calciphylaxis Who Require Anticoagulation: A Retrospective Analysis. Am J Nephrol 2018; 48: 168–171. [DOI] [PubMed] [Google Scholar]
- 115.Cheung KL, Bouchard BA, Cushman M. Venous thromboembolism, factor VIII and chronic kidney disease. Thromb Res 2018; 170: 10–19. [DOI] [PubMed] [Google Scholar]
- 116.Vanhove T, Spriet I, Annaert P, et al. Effect of the Direct Oral Anticoagulants Rivaroxaban and Apixaban on the Disposition of Calcineurin Inhibitors in Transplant Recipients. Ther Drug Monit 2017; 39: 77–82. [DOI] [PubMed] [Google Scholar]
- 117.Brodsky SV, Satoskar A, Chen J, et al. Acute kidney injury during warfarin therapy associated with obstructive tubular red blood cell casts: a report of 9 cases. Am J Kidney Dis 2009; 54: 1121–1126. [DOI] [PubMed] [Google Scholar]
- 118.Glassock RJ. Anticoagulant-Related Nephropathy: It’s the Real McCoy. Clin J Am Soc Nephrol 2019; 14: 935–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Brodsky S, Eikelboom J, Hebert LA. Anticoagulant-Related Nephropathy. J Am Soc Nephrol 2018; 29: 2787–2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Piran S, Traquair H, Chan N, et al. Incidence and risk factors for acute kidney injury in patients with excessive anticoagulation on warfarin: a retrospective study. J Thromb Thrombolysis 2018; 45: 557–561. [DOI] [PubMed] [Google Scholar]
- 121.de Aquino Moura KB, Behrens PMP, Pirolli R, et al. Anticoagulant-related nephropathy: systematic review and meta-analysis. Clin Kidney J 2019; 12: 400–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Brodsky SV, Nadasdy T, Rovin BH, et al. Warfarin-related nephropathy occurs in patients with and without chronic kidney disease and is associated with an increased mortality rate. Kidney Int 2011; 80: 181–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Caldeira D, Goncalves N, Pinto FJ, et al. Risk of renal failure with the non-vitamin K antagonist oral anticoagulants: systematic review and meta-analysis. Pharmacoepidemiol Drug Saf 2015; 24: 757–764. [DOI] [PubMed] [Google Scholar]
- 124.Chan YH, See LC, Tu HT, et al. Efficacy and Safety of Apixaban, Dabigatran, Rivaroxaban, and Warfarin in Asians With Nonvalvular Atrial Fibrillation. J Am Heart Assoc 2018; 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yao X, Tangri N, Gersh BJ, et al. Renal Outcomes in Anticoagulated Patients With Atrial Fibrillation. J Am Coll Cardiol 2017; 70: 2621–2632. [DOI] [PubMed] [Google Scholar]
- 126.Bohm M, Ezekowitz MD, Connolly SJ, et al. Changes in Renal Function in Patients With Atrial Fibrillation: An Analysis From the RE-LY Trial. J Am Coll Cardiol 2015; 65: 2481–2493. [DOI] [PubMed] [Google Scholar]
- 127.Fordyce CB, Hellkamp AS, Lokhnygina Y, et al. On-Treatment Outcomes in Patients With Worsening Renal Function With Rivaroxaban Compared With Warfarin: Insights From ROCKET AF. Circulation 2016; 134: 37–47. [DOI] [PubMed] [Google Scholar]
- 128.Poterucha TJ, Goldhaber SZ. Warfarin and Vascular Calcification. Am J Med 2016; 129: 635 e631–634. [DOI] [PubMed] [Google Scholar]
- 129.Kruger PC, Robinson MA, Xu K, et al. Assessing renal function in patients receiving DOACs: Cockcroft-Gault versus estimated glomerular filtration rate. Thromb Res 2017; 157: 165–166. [DOI] [PubMed] [Google Scholar]
- 130.Schwartz JB. Potential Effect of Substituting Estimated Glomerular Filtration Rate for Estimated Creatinine Clearance for Dosing of Direct Oral Anticoagulants. J Am Geriatr Soc 2016; 64: 1996–2002. [DOI] [PubMed] [Google Scholar]
- 131.Ganeval D, Fischer AM, Barre J, et al. Pharmacokinetics of warfarin in the nephrotic syndrome and effect on vitamin K-dependent clotting factors. Clin Nephrol 1986; 25: 75–80. [PubMed] [Google Scholar]
- 132.Obata F, Abe H, Murakami T, et al. Direct oral anticoagulant successfully used to treat an adult nephrotic patient complicated with portal vein thrombosis. CEN Case Rep 2019; 8: 134–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Dupree LH, Reddy P. Use of rivaroxaban in a patient with history of nephrotic syndrome and hypercoagulability. Ann Pharmacother 2014; 48: 1655–1658. [DOI] [PubMed] [Google Scholar]
- 134.Sasaki Y, Raita Y, Uehara G, et al. Carotid thromboembolism associated with nephrotic syndrome treated with dabigatran. Case Rep Nephrol Urol 2014; 4: 42–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Holbrook AM, Pereira JA, Labiris R, et al. Systematic overview of warfarin and its drug and food interactions. Arch Intern Med 2005; 165: 1095–1106. [DOI] [PubMed] [Google Scholar]
- 136.Gelosa P, Castiglioni L, Tenconi M, et al. Pharmacokinetic drug interactions of the non-vitamin K antagonist oral anticoagulants (NOACs). Pharmacol Res 2018; 135: 60–79. [DOI] [PubMed] [Google Scholar]
- 137.Doki K, Neuhoff S, Rostami-Hodjegan A, et al. Assessing Potential Drug-Drug Interactions Between Dabigatran Etexilate and a P-Glycoprotein Inhibitor in Renal Impairment Populations Using Physiologically Based Pharmacokinetic Modeling. CPT Pharmacometrics Syst Pharmacol 2019; 8: 118–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Chang SH, Chou IJ, Yeh YH, et al. Association Between Use of Non-Vitamin K Oral Anticoagulants With and Without Concurrent Medications and Risk of Major Bleeding in Nonvalvular Atrial Fibrillation. JAMA 2017; 318: 1250–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Goldenberg NA, Takemoto CM, Yee DL, et al. Improving evidence on anticoagulant therapies for venous thromboembolism in children: key challenges and opportunities. Blood 2015; 126: 2541–2547. [DOI] [PubMed] [Google Scholar]
- 140.Monagle P, Lensing AWA, Thelen K, et al. Bodyweight-adjusted rivaroxaban for children with venous thromboembolism (EINSTEIN-Jr): results from three multicentre, single-arm, phase 2 studies. Lancet Haematol 2019; 6: e500–e509. [DOI] [PubMed] [Google Scholar]
- 141.Halton JML, Albisetti M, Biss B, et al. Phase IIa study of dabigatran etexilate in children with venous thrombosis: pharmacokinetics, safety, and tolerability. J Thromb Haemost 2017; 15: 2147–2157. [DOI] [PubMed] [Google Scholar]
- 142.Yee DL, O’Brien SH, Young G. Pharmacokinetics and pharmacodynamics of anticoagulants in paediatric patients. Clin Pharmacokinet 2013; 52: 967–980. [DOI] [PubMed] [Google Scholar]
- 143.Maas H, Gropper S, Huang F, et al. Anticoagulant Effects of Dabigatran in Paediatric Patients Compared with Adults: Combined Data from Three Paediatric Clinical Trials. Thromb Haemost 2018; 118: 1625–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Attard C, Monagle P, Kubitza D, et al. The in-vitro anticoagulant effect of rivaroxaban in neonates. Blood Coagul Fibrinolysis 2014; 25: 237–240. [DOI] [PubMed] [Google Scholar]
- 145.Beyer-Westendorf J, Gehrisch S. Phamacokinetics of rivaroxaban in adolescents. Hamostaseologie 2014; 34: 85–87. [DOI] [PubMed] [Google Scholar]