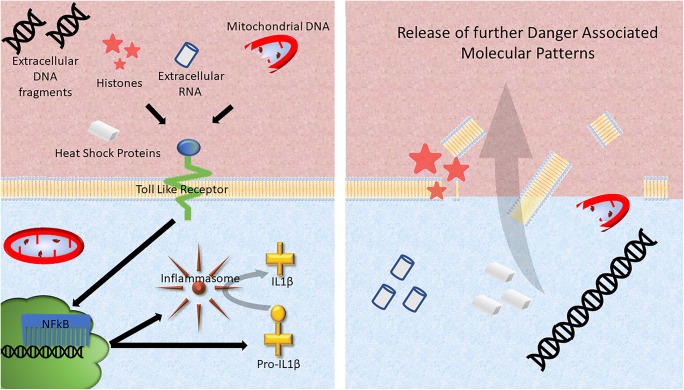
Fig. 1.
During necrosis, the cell membrane breaks down and the fragmented intracellular contents enter the extracellular space. Here, certain components such as DNA, heat shock proteins and histones can act as danger-associated molecular patterns (DAMPs), further activating intracellular cell death pathways via toll-like receptor (TLR). TLRs trigger an intracellular signaling cascade that culminates in the translocation of NF-κB to the nucleus where it stimulates the synthesis of proteins including the components of the inflammasome complex, pro-IL-1β and pro-caspase-1. Inflammasome activation is dependent on a secondary signal. Extracellular DAMPs such as ATP can trigger K+ efflux, triggering the formation and activation of the inflammasome complex. This facilitates autocatalytic activation of pro-caspase-1 into caspase-1 and cleavage of the pro-IL-1β into IL-1β. The active caspases contribute to pyroptosis and cell membrane rupture. The subsequent release of intracellular contents including DNA into the extracellular space results in this debris functioning as additional DAMPs, thereby propagating a wave of cellular injury and death