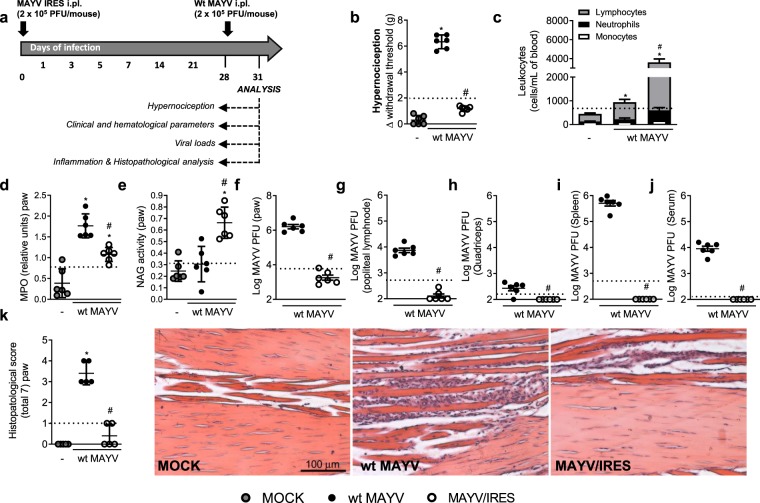
Figure 5.
Vaccination with MAYV/IRES strain protects mice from wild-type MAYV challenge. Six-week-old BALB/c mice were inoculated or not with MAYV/IRES (2 × 105 PFU/50 μL, i.pl.) and 28 days later mice were challenged with 2 × 105 PFU/50 μL of WT-MAYV at the same hind paw. Three days later, several analyses were performed. (a) Experimental design. (b) Mechanical hypernociception was assessed at day 3 after virus inoculation, as described in figure legend 1. (c) Differential cell counts on blood were represented as number of leukocytes, mononuclear cells and neutrophils normalized on % of total cells counts. (d) Neutrophil influx to the hind foot was measured indirectly by evaluation of MPO activity. (e) Macrophage influx to the hind foot was measured indirectly by analysis of NAG activity. (f–j) Plaque assay analysis of hind paw (f) PLN (g), quadriceps muscle (h), spleen (i) and serum (j). Results are shown as the log of PFU per/g of tissue or PFU per/mL of serum. (k) Shows semi-quantitative analysis (histopathological score) after Hematoxylin & Eosin staining of hind paw sections of control and MAYV-infected mice three days after wt MAYV inoculation. Representative pictures from hind paw sections. MOCK- not-infected. Results were expressed as median (f–j) or mean ± SEM (a–e and k) and are representative of two experiments. Original magnification: 200×. Scale bar: 100 μm. * for p < 0.05 when compared to control uninfected mice (MOCK). #p < 0.05 when compared to naïve wt MAYV infected group, as assessed by one-way ANOVA followed by Newman-Keuls post-test. Dashed lines are representative of naïve mice, which received MAYV/IRES.