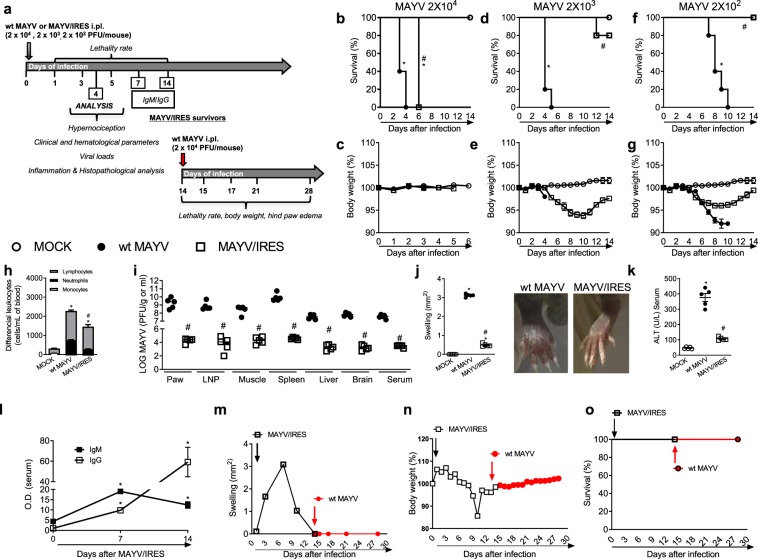
Figure 8.
Immunogenicity and efficacy of the live attenuated MAYV/IRES vaccine in A129 mice. (a) Experimental design. (b–g) 8-week-old A129 mice were inoculated with 2 × 104, 2 × 103 or 2 × 102 PFU/50 μL of either MAYV/IRES or wt MAYV strains and lethality rates (b,d,f) and body weight (c,e,g) analyzed every 12 hours until day 14 post-virus inoculation. Results are shown as % survival or body weight. Intermediary inoculum (2 × 103 PFU/mouse) was chosen for further experiments (h–o). Analysis were conducted 4 d.p.i. h) Total and differential cell counts of inflammatory cells in the blood. (i) Plaque assay analysis of hind paw, PLN, quadriceps muscle, spleen, liver, brain and serum. Results are shown as the log of PFU per/g of tissue or PFU per/mL of serum. (j) Footpad swelling was assessed by measuring the height and width of the perimetatarsal area of the hind foot. Results are shown as mm2. (k) ALT levels in serum. Results are show as U/L of serum. (l) Anti-MAYV IgM and IgG titers in serum collected 0, 7 and 14 d.p.i. Results are expressed as O.D. (m) Footpad swelling was assessed by measuring the height and width of the perimetatarsal area of the hind foot before and after wt MAYV challenge. Results are shown as mm2. (n) % of body weight. (o) % of survival. Results were expressed as median (i) or mean ± SEM (b–h and j–o) and are representative of two experiments. *p < 0.05 when compared to control uninfected mice (MOCK). #p < 0.05 when compared to naïve wt MAYV infected group, as assessed by two-way (b–g) or one-way ANOVA followed by Newman-Keuls post-test (h–o).