Graphical abstract
The complexes [Cu2(o-NO2–C6H4COO)4(PNO)2] (1), [Cu2(C6H5COO)4(2,2′-BPNO)]n (2), [Cu2(C6H5COO)4(4,4′-BPNO)]n (3), [Cu(p-OH–C6H4COO)2(4,4′-BPNO)2·H2O]n (4), [Cu(NicoNO)2·2H2O]n·4nH2O (5) where (PNO = pyridine N-oxide, 2,2′-BPNO = 2,2′-bipyridyl-N,N′-dioxide, 4,4′-BPNO = 4,4′-bipyridyl-N,N′-dioxide, NicoNO = nicotinic acid N-oxide) are prepared and characterized and their magnetic properties are studied as a function of temperature.
Keywords: Coordination polymer; Copper(II) complexes; 2,2′-Bipyridyl-N,N′-dioxide; 4,4′-Bipyridyl-N,N′-dioxide; Crystal structure; Magnetochemistry
Abstract
The complexes [Cu2(o-NO2–C6H4COO)4(PNO)2] (1), [Cu2(C6H5COO)4(2,2′-BPNO)]n (2), [Cu2(C6H5COO)4(4,4′-BPNO)]n (3), [Cu(p-OH–C6H4COO)2(4,4′-BPNO)2·H2O]n (4), (where PNO = pyridine N-oxide, 2,2′-BPNO = 2,2′-bipyridyl-N,N′-dioxide, 4,4′-BPNO = 4,4′-bipyridyl-N,N′-dioxide) are prepared and characterized and their magnetic properties are studied as a function of temperature. Complex 1 is a discrete dinuclear complex while complexes 2–4 are polymeric of which 2 and 3 have paddle wheel repeating units. Magnetic susceptibility measurements from polycrystalline samples of 1–4 revealed strong antiferromagnetic interactions within the {Cu2}4+ paddle wheel units and no discernible interactions between the units. The complex 5, [Cu(NicoNO)2·2H2O]n·4nH2O, in which the bridging ligand to the adjacent copper(II) ions is nicotinate N-oxide (NicoNO) the transmitted interaction is very weakly antiferromagnetic.
1. Introduction
Interest in heterocyclic aromatic N-oxides has flourished because of their practical impact on biological activity [1]. A number of N-oxide derivatives are known to show herbicidal activity [2], inhibitory activity [3] such as against feline coronavirus and human SARS-CoV, as well as antibiotic properties, as for example 2-n-heptyl-4-hydroxyquinoline N-oxide [4], [5]. This latter N-oxide is also known for mediating proton transfer across the mitochondrial membrane as well as for inhibiting electron transport across cell membranes in complex form [4], [6]. Moreover, researchers have reported several aromatic N-oxide derivatives capable of forming biologically active metal complexes [7], [8]. Apart from these, coordination complexes of aromatic N-oxides are known to have extensive applications as magnetic materials [7], [9], [10], [11], [12], in catalysis [13], [14], [15], [16], [17], [18], [19] as well as in supramolecular systems [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31].
The N-oxides can act as monodentate or bridging ligands (Scheme 1 ) and we have shown that they can lead to polymeric complexes even with simple mono carboxylic acids [32], [33], [34], [35], [36]. It has been found that in the case of pyridine N-oxide bridged coordination polymers the metal–metal separations lie in the 3.6–3.7 Å range and they exhibit antiferromagnetic exchange [36], [37], [38], [39], [40], [41]. In this present study we have decided to undertake the study of copper(II) N-oxide complexes for two reasons: (i) copper(II) N-oxide complexes have been shown to exhibit biological activity [7], [8] and (ii) to study the antiferromagnetic properties in paddle wheel containing copper complexes.
Scheme 1.
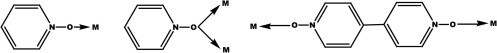
Some common binding mode of N-oxides.
In general copper(II) carboxylates prefers paddle wheel geometry and in such complexes axial positions are occupied by solvents or by ancillary ligands. Thus, by use of bidentate spacer ligands to connect these paddle wheel units the distance of separation between the paddle wheel cores may be controlled. As shown in Scheme 2 such distance will be different for different ligands used (d1 > d2), which in turn may have significant effects on their magnetic exchange properties. However, in the case of 2,2′-bipyridyl-N,N′-dioxide there is other possibilities such as chelation, which need to be overcome so that polymeric structures are preferred. With the intention of studying magnetostructural correlations with some copper(II) complexes of such polymeric complexes we have synthesized a number of coordination complexes/polymers of copper(II) aromatic N-oxides having carboxylate as the anionic ligand and studied their magnetic properties as a function of temperature. We have studied complexes of N-oxide ligands such as pyridine N-oxide, 2,2′-bipyridyl-N,N′-dioxide, 4,4′-bipyridyl-N,N′-dioxide and nicotinic acid N-oxide to compare the structural as well as magnetic features of the different complexes formed. All the complexes reported here are characterized by conventional spectroscopic techniques as well as with single-crystal X-ray diffraction.
Scheme 2.
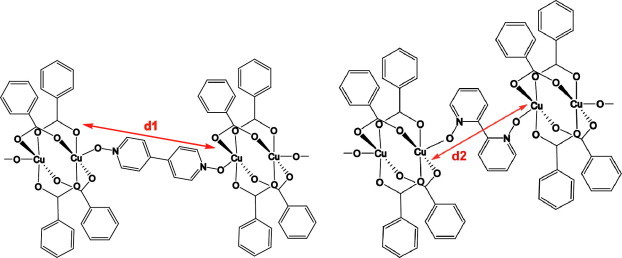
The separation of paddle wheel units by N-oxide connectors.
2. Experimental
The complexes [Cu2(o-NO2–C6H4COO)4(PNO)2] (1), [Cu2(C6H5COO)4(2,2′-BPNO)]n (2), [Cu2(C6H5COO)4(4,4′-BPNO)]n (3), [Cu(p-OH–C6H4COO)2(4,4′-BPNO)2·H2O]n (4), [Cu(NicoNO)2·2H2O]n·4nH2O (5) were prepared through solution phase synthetic route (where PNO = pyridine N-oxide; 2,2′-BPNO = 2,2′-bipyridyl-N,N′-dioxide; 4,4′-BPNO = 4,4′-bipyridyl-N,N′-dioxide; NicoNO = nicotinate N-oxide) .
2.1. Synthesis of 1, 2
To a solution of o-nitrobenzoic acid (1 mmol, 0.167 g) in methanol (15 mL) copper(II) actetate monohydrate (0.5 mmol, 0.100 g) was added and stirred for 10 min. To this reaction mixture pyridine N-oxide (1 mmol, 0.095 g) was added with constant stirring at room temperature. A small amount (≈5 mL) of toluene was then added to dissolve the precipitate that appeared after addition of pyridine N-oxide. Diffraction quality crystals were collected after 7 days and dried in air. Yield of the pure crystalline complex was found to be >70%. IR (KBr, cm−1): 3421 (bw), 3084 (w), 1643 (s), 1614 (s), 1575 (m), 1529 (s), 1467 (m), 1410 (s), 1369 (m), 1351 (m), 1227 (m), 839 (m), 702 (m). UV–Vis λ max (methanol): 733 nm; ε = 108 M−1 cm−1.
Complex 2 was prepared with a similar procedure and crystalline pure products with 55% yield were obtained. IR (KBr, cm−1): 3445 (bw), 3085 (w), 1618 (s), 1597 (m), 1572 (s), 1410 (s), 1228 (s), 719 (m). UV–Vis λ max (methanol): 715 nm; ε = 99 M−1 cm−1.
2.2. Synthesis of 3
Single-crystals of 3 were grown by layering a solution of 4,4′-bipyridyl-N,N′-dioxide (0.25 mmol, 0.045 g) in water (3 mL) over a reaction mixture containing copper(II) acetate monohydrate (0.25 mmol, 0.050 g) and benzoic acid (0.5 mmol, 0.061 g) in toluene: methanol (3:1, 10 mL). IR (KBr, cm−1): 3421 (bw), 3110 (m), 1633 (s), 1614 (s), 1572 (m), 1462 (m), 1403 (s), 1230 (s), 1175 (w), 840 (m), 716 (m). UV–Vis λ max (methanol): 711 nm; ε = 246 M−1 cm−1.
2.3. Synthesis of 4
To a solution of p-hydroxybenzoic acid (1 mmol, 0.138 g) in methanol (15 mL) copper(II) acetate monohydrate (0.5 mmol, 0.100 g) was added and stirred for 10 min. To this reaction mixture 4,4′-bipyridyl-N,N′-dioxide (0.5 mmol, 0.095 g) was added with constant stirring at room temperature. A small amount (≈5 mL) of dimethylformamide was then added to dissolve the precipitate that appeared after addition of 4,4′-bipyridyl-N,N′-dioxide. Diffraction quality crystals were collected after 12 days. Yield of the pure crystalline complex was found to be ≈40%. IR (KBr, cm−1): 3116 (bm), 1614 (s), 1598 (s), 1574 (s), 1475 (m), 1372 (s), 1225 (s), 1029 (m), 838 (m), 785 (m). UV–Vis λ max (methanol): 703 nm; ε = 117 M−1 cm−1.
2.4. Synthesis of 5
To a solution of nicotinic acid N-oxide (1 mmol, 0.139 g) in methanol (15 mL) methanolic solution of copper(II) acetate monohydrate (0.5 mmol, 0.100 g) (10 mL) was added. After stirring this reaction mixture for about 30 min precipitation appeared. The precipitate was dissolved by adding 5–10 mL of water to it. The reaction mixture was then allowed to stir for another 30 min and then kept for crystallization. Crystals of 5 were obtained after about 7 days. Yield of the crystalline complexes were found to be >80%. IR (KBr, cm−1): 3403 (bm), 3081 (m), 1657 (m), 1591 (s), 1557 (s), 1477 (w), 1387 (s), 1219 (m), 731 (m). UV–Vis λ max (methanol): 705 nm; ε = 249 M−1 cm−1.
2.5. X-ray crystallography
The X-ray crystallographic data were collected at 296 K with Mo Kα radiation (λ = 0.71073 Å) using a Bruker Nonius SMART CCD diffractometer equipped with graphite monochromator. The smart software was used for data collection and also for indexing the reflections and determining the unit cell parameters; the collected data were integrated using saint software. The structures were solved by direct methods and refined by full-matrix least-squares calculations using shelxtl software. All the non-H-atoms were refined in the anisotropic approximation against F 2 of all reflections. The H-atoms, except those attached to O were placed at their calculated positions and refined in the isotropic approximation; those attached to hetero-atoms (O) were located in the difference Fourier maps, and refined with isotropic displacement coefficients. The crystallographic parameters of three complexes are given in Table 1 . The crystallographic parameters for the complex 3 are not that satisfactory; also the crystal structure of 5 is already reported and hence not included in the Table 1.
Table 1.
Crystallographic parameters of the complexes 1, 2 and 4.
| Compound number | 1 | 2 | 4 |
|---|---|---|---|
| Formulae | C38H26Cu2N6O18 | C19H14CuNO5 | C24H20CuN2O9 |
| Formula weight | 981.73 | 399.85 | 543.96 |
| Crystal system | monoclinic | monoclinic | monoclinic |
| Space group | P21/c | C2/c | Cc |
| a (Å) | 11.3721(4) | 20.6947(2) | 18.9930(7) |
| b (Å) | 10.1756(3) | 10.10310(10) | 15.8109(7) |
| c (Å) | 19.7779(6) | 19.1416(2) | 7.7635(3) |
| α (°) | 90.00 | 90.00 | 90.00 |
| β (°) | 117.384(2) | 118.17(10) | 107.791(4) |
| γ (°) | 90.00 | 90.00 | 90.00 |
| V (Å3) | 2032.20(11) | 755.32(13) | 2219.86(15) |
| Z | 2 | 8 | 4 |
| Density (Mg m−3) | 1.604 | 1.506 | 1.628 |
| Absolute coefficient (mm−1) | 1.133 | 1.267 | 1.045 |
| F(0 0 0) | 996 | 1632 | 1116 |
| Total number of reflections | 21419 | 18542 | 12347 |
| Reflections [I > 2σ(I)] | 3672 | 3105 | 5104 |
| Maximum θ (°) | 25.50 | 25.00 | 28.93 |
| Ranges (h, k, l) | −13 ⩽ h ⩽ 13, −12 ⩽ k ⩽ 10, −24 ⩽ l ⩽ 24 | −23 ⩽ h ⩽ 24, −11 ⩽ k ⩽ 12, −22 ⩽ l ⩽ 22 | −23 ⩽ h ⩽ 25, −21 ⩽ k ⩽ 21, −10 ⩽ l ⩽ 10 |
| Completeness to 2θ (%) | 97.0 | 99.9 | 99.1 |
| Data/restraints/parameters | 3672/0/289 | 3105/0/235 | 5104/0/332 |
| Goodness-of-fit (GOF) (F2) | 1.081 | 1.052 | 1.082 |
| R indices [I > 2σ(I)] | R1 = 0.0349, wR2 = 0.0861 | R1 = 0.0223, wR2 = 0.0614 | R1 = 0.0451, wR2 = 0.1113 |
| R indices (all data) | R1 = 0.0459, wR2 = 0.0919 | R1 = 0.0258, wR2 = 0.0636 | R1 = 0.0558, wR2 = 0.1179 |
3. Magnetic measurements
Variable temperature magnetic susceptibility measurements were carried out on polycrystalline samples of 1–3 (5–300 K) and 5 (2–300 K) using a Quantum Design MPMS SQUID susceptometer under magnetic fields of 0.5 (1–3) and 0.1 (5) T. Diamagnetic corrections for the complexes were estimated from Pascal′s constants. The magnetic susceptibility of 1–3 has been computed by exact calculation of the energy levels associated with the spin Hamiltonian through diagonalization of the full-matrix with a general program for axial symmetry [42]. Least-squares fits were accomplished with an adapted version of the function-minimization program minuit [43]. The error-factor R is defined as , where N is the number of experimental points.
4. Results and discussion
Copper(II) acetate reacts with o-nitrobenzoic acid and pyridine N-oxide resulting in the formation of the complex 1, [Cu2(o-NO2–C6H4COO)4(PNO)2]. It has a dinuclear paddle wheel structure with two terminal pyridine N-oxide coordination (Fig. 1 ). The complex exhibits characteristic IR stretching frequencies at 1614 and 1467 cm−1 due to the carboxylate stretching, the N-oxo stretching appears at 1227 cm−1. The single-crystal X-ray analysis reveals that 1 crystallizes in the monoclinic P21/c space group. The asymmetric unit of 1 consists of two o-nitrobenzoate ligands and one pyridine N-oxide ligand coordinated to a copper(II) centre. In the dinuclear molecule each of the copper centres (Cu1) adopts a square-pyramidal geometry with the four oxygen atoms O1, O2, O5 and O9 from four different carboxylate ligands residing at the corners of the basal plane. The apical position is occupied by the N-oxo, O8, of the pyridine N-oxide ligand. The basal atoms are almost coplanar and the Cu1 is positioned 0.224 Å above the basal plane. The bond lengths for the basal bonds (Cu–O1, Cu–O2, Cu–O5, Cu–O9) lie in the range 1.963–1.993 Å, while that of the axial bond (Cu1–O8) is 2.116 Å and the Cu–Cu separation is 2.686 Å which is normal for other copper complexes reported.
Fig. 1.
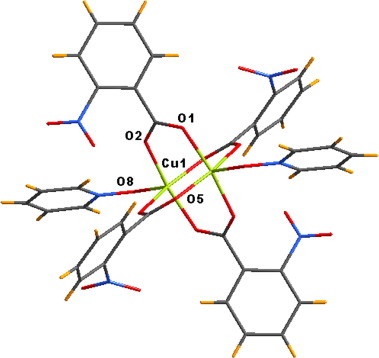
Dinuclear paddle wheel unit of 1.
With the intention of linking together the dinuclear paddle wheel units as formed in case of 1 we have reacted copper(II) acetate, benzoic acid with the ′spacer′ ligand 2,2′-bipyridyl-N,N′-dioxide. This ligand being a bidentate ligand resulted in the formation of the 1D coordination polymer 2 with the composition [Cu2(C6H5COO)4(2,2′-BPNO)]n. The 2,2′-bipyridyl-N,N′-dioxide ligand can act either as a chelating or as bridging ligand. This is possible because of the free rotation around the C–C single bond between the two rings of 2,2′-bipyridyl-N,N′-dioxide. In other words it can be said that 2,2′-bipyridyl-N,N′-dioxide can coordinate to metal centres either in a cis or a trans configuration as shown in Scheme 3 .
Scheme 3.
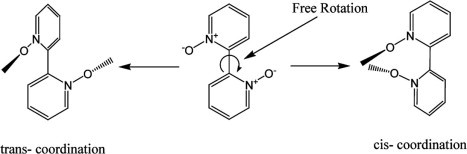
Two conformations of 2,2′-bipyridyl-N,N′-dioxide towards coordination.
There are a few reports available on the chelating coordination mode of 2,2′-bipyridyl-N,N′-dioxide [43], [44], [45]. However, in case of the coordination polymer 2, the 2,2′-bipyridyl-N,N′-dioxide coordinates in the trans-fashion to bridge two paddle wheel units of copper(II) benzoate. The crystal structure of 2 is shown in Fig. 2 . It crystallizes in the monoclinic space group C2/c and each asymmetric unit of 2 consists of one copper(II) atom, two benzoate ligands and half a 2,2′-bipyridyl-N,N′-dioxide molecule. Like in 1, here also each of the copper(II) centres adopts a square-pyramidal geometry, with the N-oxo of 2,2′-bipyridyl-N,N′-dioxide apically coordinated. Four of the carboxylate oxygens O1, O2, O3 and O4 occupy the corners of the basal plane, with bond lengths spanning the 1.957–1.983 Å range. The apical oxygen O5 is residing at a distance 2.171 Å from the copper(II) centre and the copper(II) centre is at a distance of 0.202 Å from the basal plane. The Cu–Cu distance in the paddle wheel unit is 2.639 Å, while the smallest Cu···Cu distance between two paddle wheel units is 8.035 Å. Moreover the two planes of the two aromatic rings of 2,2′-bipyridyl-N,N′-dioxide form an angle of 67.12° which differs significantly from those reported for the cis-coordination geometries.
Fig. 2.
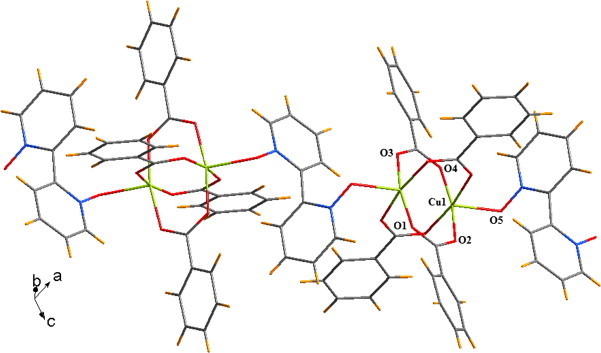
1D chain of the coordination polymer 2.
A similar reaction of copper(II) acetate monohydrate and benzoic acid with 4,4′-bipyridyl-N,N′-dioxide led to an one-dimensional coordination polymer 3 of composition [Cu2(C6H5COO)4(4,4′-BPNO)]n with the paddle wheel building blocks of copper(II) benzoate bridged through the spacer N-oxide ligand. We have evidence that the complex belongs to the triclinic space group and the structure of the coordination polymer is as shown in Fig. 3 . As in 2, in the coordination polymer 3 also the copper(II) adopts the expected tetragonal pyramidal geometry. However, the difference comes in the orientation of the 4,4′-bipyridyl-N,N′-dioxide ligand which gets distorted may be due to the geometry forced by the copper(II) ions. The two aromatic rings of the ligand are inclined at an angle 23.96° to each other, departing from the reported ones where they are mostly coplanar [10]. The separation between two nearest paddlewheel units is 12.57 Å. Some of the important bond lengths and angles are tabulated in Table 2 .
Fig. 3.
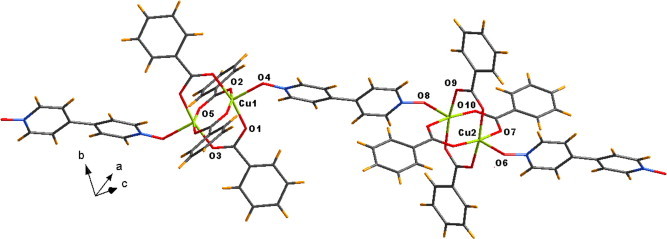
1D chain of the coordination polymer 3.
Table 2.
Selected bond lengths (Å) and bond angles (°) of the complexes 1–4.
| Bond distance (Å) | Bond angle (°) | Bond distance (Å) | Bond angle (°) | ||||
|---|---|---|---|---|---|---|---|
| For 1 | For 2 | ||||||
| Cu1 O1 | 1.9926(16) | O5 Cu1 O9 | 166.78(8) | Cu1 O1 | 1.9837(12) | O3 Cu1 O2 | 87.82(6) |
| Cu1 O2 | 1.9749(17) | O5 Cu1 O2 | 88.50(8) | Cu1 O2 | 1.9568(12) | O3 Cu1 O4 | 168.02(5) |
| Cu1 O5 | 1.9625(18) | O5 Cu1 O1 | 90.70(7) | Cu1 O3 | 1.9567(12) | O2 Cu1 O4 | 89.33(5) |
| Cu1 O8 | 2.1158(19) | O9 Cu1 O1 | 89.15(7) | Cu1 O4 | 1.9745(12) | O3 Cu1 O1 | 90.65(6) |
| Cu1 O9 | 1.9646(18) | O2 Cu1 O1 | 167.12(7) | Cu1 O5 | 2.1715(11) | O2 Cu1 O1 | 168.26(5) |
| O5 Cu1 O8 | 92.32(8) | O4 Cu1 O1 | 89.77(5) | ||||
| O9 Cu1 O8 | 100.85(8) | O3 Cu1 O5 | 95.27(5) | ||||
| O2 Cu1 O8 | 97.22(8) | O2 Cu1 O5 | 104.72(5) | ||||
| O1 Cu1 O8 | 95.66(8) | O4 Cu1 O5 | 96.71(5) | ||||
| O1 Cu1 O5 | 87.01(4) | ||||||
| For 3 | For 4 | ||||||
| Cu1 O1 | 1.942(13) | O3 Cu1 O5 | 90.6(5) | Cu1 O1 | 1.908(3) | O1 Cu1 O3 | 175.96(11) |
| Cu1 O2 | 1.971(12) | O1 Cu1 O5 | 88.0(5) | Cu1 O3 | 1.910(4) | O1 Cu1 O6 | 92.20(13) |
| Cu1 O3 | 1.942(12) | O3 Cu1 O2 | 90.6(5) | Cu1 O6 | 1.966(3) | O3 Cu1 O6 | 90.38(13) |
| Cu1 O4 | 2.137(12) | O3 Cu1 O4 | 94.6(5) | Cu1 O5 | 2.046(3) | O1 Cu1 O5 | 87.28(13) |
| Cu1 O5 | 1.962(11) | O1 Cu1 O4 | 96.7(5) | Cu1 O7 | 2.527 | O3 Cu1 O5 | 90.97(14) |
| Cu2 O6 | 1.950(11) | O5 Cu1 O4 | 100.3(5) | O6 Cu1 O5 | 165.96(15) | ||
| Cu2 O7 | 1.970(13) | O2 Cu1 O4 | 91.5(5) | O3 Cu1 O7 | 88.74 | ||
| Cu2 O8 | 2.119(11) | O9 Cu2 O10 | 88.8(5) | O1 Cu1 O7 | 87.94 | ||
| Cu2 O9 | 1.935(12) | O6 Cu2 O10 | 90.8(5) | O5Cu1 O7 | 99.10 | ||
| Cu2 O10 | 1.956(12) | O9 Cu2 O7 | 91.1(6) | O6Cu1 O7 | 94.91 | ||
| O6 Cu2 O7 | 86.7(6) | ||||||
| O9 Cu2 O8 | 98.0(5) | ||||||
| O6 Cu2 O8 | 93.1(5) | ||||||
| O10 Cu2 O8 | 100.4(5) | ||||||
| O7 Cu2 O8 | 92.5(5) | ||||||
The reaction between copper(II) acetate monohydrate, p-hydroxybenzoic acid and 4,4′-bipyridyl-N,N′-dioxide leads to the formation of another 1D coordination polymer 4 with composition [Cu(p-OH–C6H4COO)2(4,4′-BPNO)2·H2O]n. It differs significantly from the earlier two polymers in that unlike 2 and 3, 4 does not possess paddle wheel units, rather it forms mononuclear copper(II) units bridged through the spacer ligand. The structure of 4 is shown in Fig. 4 which shows a square-pyramidal geometry around the copper centre. Two of the coordination come from two benzoate ligands, two from the 4,4′-bipyridyl-N,N′-dioxide and the rest from the aquo-ligand. The oxygen O7 from 4,4′-bipyridyl-N,N′-dioxide occupies the apical position with a Cu–O bond distance of 2.527 Å. Here, the distance between two nearest copper(II) centres bridged by 4,4′-bipyridyl-N,N′-dioxide is 12.007 Å. The one-dimensional chains interact through the hydrogen bonds O6–H6A···O2 and O6–H6B···O4 between aquo-groups and the carboxylate oxygen and O8–H8···O7, between the hydroxy group on the aromatic carboxylate ring and one of the N-oxo groups. The hydrogen bonded self assembly is shown in the Fig. 4b along with the coordination environment around Cu1 in Fig. 4a.
Fig. 4.
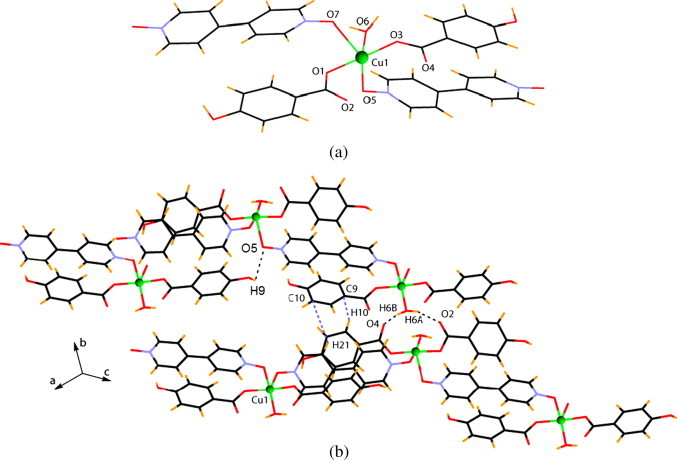
(a) Coordination environment around Cu in 4, (b) self assembly of 4 showing various short range interactions.
A completely different product is found to be formed in the reaction between copper(II) acetate and nicotinic acid N-oxide where six coordination rather than five coordination of copper(II) is observed. The reaction yields the one-dimensional coordination polymer 5 with the composition [Cu(NicoNO)2·2H2O]n·4nH2O crystallizing in the monoclinic space group P21/c. The crystal structure of 5 has already been reported [47] with space group P21 and then correctly reinterpreted by Clemente [48] and is not discussed here. However the magnetic measurement for 5 is carried out and discussed in the next section. It should be noted that the structure contains as many as four water molecules per copper core. These water molecules are strongly hydrogen bonded to each other forming net-like structures of hexameric aqua-units as shown in Fig. 5 a. These aqua-nets surround the one-dimensional chain of the metal complex separating them from each other as shown in Fig. 5b. This makes a layered structure comprising of polymeric chains of the metal complex embedding a layered sheets of infinite chains like structures of water.
Fig. 5.
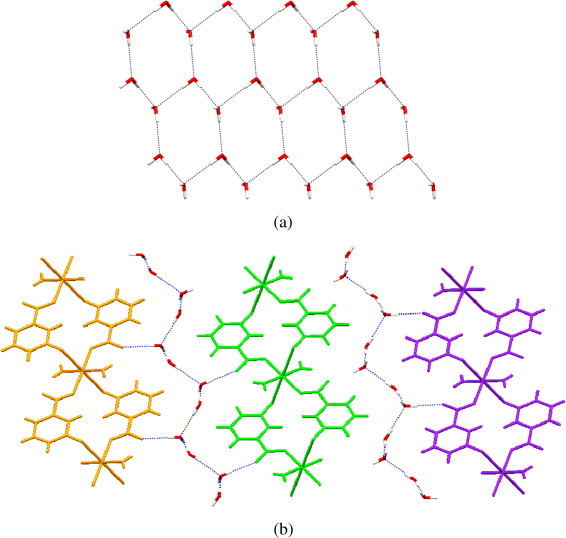
(a) Hexameric aqua-nets formed by the aqua-groups in 5. (b) The one-dimensional chains of coordination polymer separated by the aqua-nets.
At room temperature the complexes 1–3 are ESR silent; this could be due to antiferromagnetic coupling between the two copper(II) centres within the paddle wheel units. The ESR spectrum of 4 shows a well resolved four line spectra typical of copper(II) monomeric units expected for the coupling of the electron with the nuclear spin. On the other hand the ESR spectrum of complex 5 is axial in character (please refer to Supplementary material).
5. Magnetic properties
Magnetic susceptibility data for complexes 1–3 are shown in Fig. 6 . For all three complexes, the χMT product at 300 K is 0.44–0.45 cm3 mol−1 K, significantly lower than the values predicted for two non-interacting S = 1/2 spins (0.82 cm3 mol−1 K, g = 2.1), suggesting the interplay of antiferromagnetic interactions. This conclusion is corroborated by the decrease of the χMT products of all three complexes upon cooling. These are stabilized below ∼50 K, forming plateaus of different values. This indicates the presence of paramagnetic impurities, of different fractions for each complex.
Fig. 6.
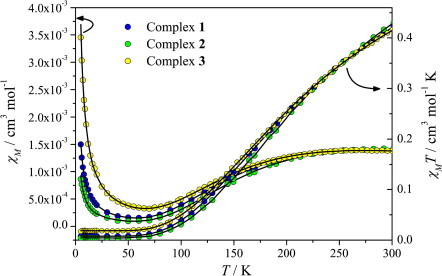
χM vs. T and χMT vs. T experimental data for complexes 1–3 and calculated curves according to the model described in the text.
For the analysis of the magnetic data a simple isotropic exchange model was employed, according to the Hamiltonian:
| (1) |
In this model we also took into account a fraction ρ of S = 1/2 paramagnetic impurity following the Curie law.
Fits to these data using this model were of good quality, yielding best-fit parameters J = −159 cm−1, g = 2.07, ρ = 2.0%, R = 2.0 × 10−4 (complex 1), J = −168 cm−1, g = 2.13, ρ = 1.2%, R = 1.2 × 10−3 (complex 2) and J = −157 cm−1, g = 2.04, ρ = 4.9%, R = 2.2 × 10−4 (complex 3). These values fall in the range of values previously determined for paddlewheel dicopper(II) carboxylate complexes [44], [45], [46].
The quality of the fits does not allow us the incorporation of additional parameters, namely a mean-field correction for the determination of eventual interactions between dinuclear units in 2 and 3, without overparametrizing the problem. Therefore, we conclude that such interactions, if indeed operative, must be very weak. This is in agreement with the fact that such interactions would have to be transmitted through a superexchange pathway involving the apical coordination position of the square-pyramidal copper(II) ions; since this position occupies a non-magnetic orbital (of character), this interaction is expected to be very weak. Moreover, the size and shape of the bridging ligands is probably not favourable for the transmission of significant magnetic exchange.
Magnetic susceptibility data for complex 5 are shown in Fig. 7 . The χMT product for 5 is 0.42 cm3 mol−1 K, the value corresponding to an isolated S = 1/2 spin (g = 2.12). This remains constant upon cooling down to ∼50 K, and then decreases down to a value of 0.36 cm3 mol−1 K, indicating weak antiferromagnetic interactions.
Fig. 7.
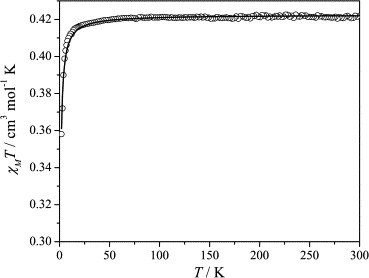
χMT vs. T experimental data for complex 5 and calculated curve according to the model described in the text.
Given the 1D structure of 5, the magnetic properties were analyzed according to the Bonner–Fisher model [49] for an equally spaced S = 1/2 Heisenberg chain. The Hamiltonian considered was:
For the fitting of the data the empirical equation derived by Hall was employed [50]:
with A = 0.25, B = 0.14995, C = 0.30094, D = 1.9862, E = 0.68854, F = 6.0626 and x = |J|/kT for the above mentioned Hamiltonian formalism.
Best-fit parameters according to this model are J = −0.71 cm−1, g = 2.12 with R = 8.2 × 10−6.
The very weak interaction can be rationalized by (i) the relatively long superexchange pathway provided by the nicotinic N-oxide ligand and (ii) the fact that magnetic exchange is transmitted between the O(3) atom of the ligand, coordinated to an axial coordination position [Cu(1)···O(3), 2.530 Å] and the O(1) atom of the ligand coordinated to an equatorial coordination position [Cu(1)···O(1), 1.955 Å]. Since the axial coordination positions occupy non-magnetic orbitals (of character), magnetic exchange transmitted through them is significantly reduced.
6. Thermogravimetric analysis
Thermal stability of the complexes 1–4 is studied. The thermograms show that all the complex/coordination polymers are thermally stable up to ∼200 °C (please refer to Supplementary materials). Thermogram of the complex 1 reveals weight loss in the range 225–275 °C which corresponds to 67.2% (calc. 67.6%) of the total weight and is accounted for loss of four o-nitrobenzoic acid. Then the weight loss due to the two pyridine N-oxide molecules takes place. For the coordination polymer 2, weight loss occurs in two steps: the first step within the range 195–250 °C corresponds to weight loss of 59.5% (calc. 58.3%) due to loss of the two benzoic acid molecules and the second step, 350–525 °C, is due to the loss of half a 2,2′-bipyridyl-N,N′-dioxide molecule (experimental 57.4% from the first step; calc. 59.1%). The coordination polymer 3 also shows a similar thermal behaviour as 2 and the coordination polymer 4 shows a continuous thermal degradation in the range 200–525 °C.
7. Conclusion
In conclusion we have synthesized and characterized copper(II) coordination polymers having aromatic N-oxide ligands as ancillary ligands. We have obtained preferential monodentate bridging binding mode of 2,2′-bipyridyl-N,N′-dioxide to form copper(II) coordination polymer rather than chelating mode, which is frequently come across. Depending on the aromatic N-oxide ligands different architectures of the coordination polymers with repeated mononuclear or dinuclear copper(II) units are observed. The paddle wheel structures of copper(II) separated by different N-oxide spacers controls their structural features. The nicotinic acid N-oxide leads to dinuclear repeated units of copper(II) to form the 1D chains with relatively larger copper(II) inter-metal separations. Interactions within the paddlewheel moieties of 1–3 were found to be strongly antiferromagnetic, with values typical of copper(II) carboxylates. However, inter-dinuclear interactions between these moieties were too weak to be detected, or non-existent. In the case of 5, in which the coordination of the bridging ligand to the adjacent copper(II) ions is both axial and equatorial, the transmitted interaction is very weakly antiferromagnetic.
Acknowledgements
The authors thank Department of Science and Technology, New Delhi, India for financial support and the author R.S. thanks Council of Scientific and Industrial Research, New Delhi, India for Junior Research Fellowship.
Footnotes
CCDC 664104, 672157, 759711, and 759712 contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif. Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.ica.2010.03.050.
Appendix A. Supplementary material
References
- 1.Albini A., Pietra S. CRC Press; Boca Raton, FL: 1991. Heterocyclic N-Oxides. [Google Scholar]
- 2.Cerecetto H., Dias E., Maio R.D., Gonzalez M., Pacce S., Saenz P., Seoane G., Suescun L., Mombru A., Fernandez G., Lema M., Villalba J. J. Agric. Food Chem. 2000;48:2995. doi: 10.1021/jf9904766. [DOI] [PubMed] [Google Scholar]
- 3.Balzarini J., Keyaerts E., Vijgen L., Vandermeer F., Stevens M., Clercq E.D., Egberink H., Ranst M.V. J. Antimicrob. Chemother. 2006;57:472. doi: 10.1093/jac/dki481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krab K., Wikstrom M. Biochem. J. 1980;186:637. doi: 10.1042/bj1860637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smirnova I.A., Hagerhall C., Konstantinov A.A., Hederstedt L. FEBS Lett. 1995;359:23. doi: 10.1016/0014-5793(94)01442-4. [DOI] [PubMed] [Google Scholar]
- 6.Furbacher P.N., Girvin M.E., Cramer W.A. Biochemistry. 1989;28:8990. doi: 10.1021/bi00449a006. [DOI] [PubMed] [Google Scholar]
- 7.Nizhnik Y.P., Hojniak A.S., Deperasińska I., Jerzykiewicz L.B., Korabik M., Hojniak M., Andreev V.P. Inorg. Chem. 2008;47:2103. doi: 10.1021/ic701413m. [DOI] [PubMed] [Google Scholar]
- 8.Puszko A., Krojcer A., Pełczynska M., Wietrzyk J., Golonka M.C., Jezierska J., Adach A., Kubiak M. J. Inorg. Biochem. 2010;104:153. doi: 10.1016/j.jinorgbio.2009.10.016. [DOI] [PubMed] [Google Scholar]
- 9.Carlin R.L., Jongh L.J.D. Chem. Rev. 1986;86:659. [Google Scholar]
- 10.Fabelo O., Pasan J., Lioret F., Julve M., Perez C.R. CrystEngComm. 2007;9:815. [Google Scholar]
- 11.Lin J.G., Su Y., Tian Z.F., Qiu L., Wen L.L., Lu Z.D., Li Y.Z., Meng Q.J. Cryst. Growth Des. 2007;7:2526. [Google Scholar]
- 12.Manna S.C., Zangrando E., Ribas J., Chaudhuri N.R. Dalton Trans. 2007:1383. doi: 10.1039/b617278d. [DOI] [PubMed] [Google Scholar]
- 13.Brasse M., Cámpora J., Palma P., Álvarez E., Cruz V., Ramos J., Reyes M.L. Organometallics. 2008;27:4711. [Google Scholar]
- 14.Cho S.H., Hwang S.J., Chang S. J. Am. Chem. Soc. 2008;130:9254. doi: 10.1021/ja8026295. [DOI] [PubMed] [Google Scholar]
- 15.Kanyiva K.S., Nakao Y., Hiyama T. Angew. Chem., Int. Ed. 2007;46:8872. doi: 10.1002/anie.200703758. [DOI] [PubMed] [Google Scholar]
- 16.Chen D.X., Ho C.M., Wu Q.Y.R., Wu P.R., Wong F.M., Wu W. Tetrahedron Lett. 2008;49:4147. doi: 10.1016/j.tetlet.2008.04.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goher M.A.S., Mautner F.A. J. Mol. Struct. 2007;846:153. [Google Scholar]
- 18.He Z., Wang Z.M., Yan C.H. CrystEngComm. 2005;7:143. [Google Scholar]
- 19.Yang B.P., Mao J.G., Dong Z.C. Inorg. Chem. Commun. 2004;7:104. [Google Scholar]
- 20.Deiters E., Bulach V., Hosseini M.W. New J. Chem. 2008;32:99. [Google Scholar]
- 21.Han J.W., Hill C.L. J. Am. Chem. Soc. 2007;129:15094. doi: 10.1021/ja069319v. [DOI] [PubMed] [Google Scholar]
- 22.Imaz I., Thillet A., Sutter J.P. Cryst. Growth Des. 2007;7:1753. [Google Scholar]
- 23.Blake A.J., Brett M.T., Champness N.R., Khlobystov A.N., Long D.L., Wilson C., Schröder M. Chem. Commun. 2001:2258. doi: 10.1039/b106348k. [DOI] [PubMed] [Google Scholar]
- 24.Roesky H.W., Andruh M. Coord. Chem. Rev. 2003;236:91. [Google Scholar]
- 25.Ma B., Gao S., Sun H.L., Xu G.X. J. Chem. Soc., Dalton Trans. 2001:130. [Google Scholar]
- 26.Deiters E., Bulach V., Hosseini M.W. Dalton Trans. 2007:4126. [Google Scholar]
- 27.Mantero D.G., Neels A., Evans H.S. Inorg. Chem. 2006;45:3287. doi: 10.1021/ic051934x. [DOI] [PubMed] [Google Scholar]
- 28.Bourne S.A., Moitsheki L.J. CrystEngComm. 2005;7:674. [Google Scholar]
- 29.Jia J., Blake A.J., Champness N.R., Hubberstey P., Wilson C., Schröder M. Inorg. Chem. 2008;47:8652. doi: 10.1021/ic800422g. [DOI] [PubMed] [Google Scholar]
- 30.Alonso A.M., Brough P., Prato M. Chem. Commun. 2007:1412. doi: 10.1039/b617835a. [DOI] [PubMed] [Google Scholar]
- 31.Reddy L.S., Babu N.J., Nangia A. Chem. Commun. 2006:1369. doi: 10.1039/b515510j. [DOI] [PubMed] [Google Scholar]
- 32.Adrabińska V.V., Janeczko E. Chem. Commun. 1999:1527. [Google Scholar]
- 33.Sarma R., Karmakar A., Baruah J.B. Inorg. Chem. 2008;47:763. doi: 10.1021/ic701639n. [DOI] [PubMed] [Google Scholar]
- 34.Sarma R., Karmakar A., Baruah J.B. Inorg. Chim. Acta. 2008;361:2081. [Google Scholar]
- 35.Sarma R., Baruah J.B. Inorg. Chim. Acta. 2009;362:1681. [Google Scholar]
- 36.Sarma R., Baruah J.B. Polyhedron. 2009;28:453. [Google Scholar]
- 37.Sarma R., Perumal A., Baruah J.B. J. Coord. Chem. 2009;62:1513. [Google Scholar]
- 38.Carlin R.L., Block R. Proc. Indian Acad. Sci. (Chem. Sci.) 1987;98:79. [Google Scholar]
- 39.Schake A.R., Vincent J.B., Li Q., Boyd P.D.W., Folting K., Huffman J.C., Hendrickson D.N., Christou G. Inorg. Chem. 1989;28:1915. [Google Scholar]
- 40.Christou G. Polyhedron. 2005;24:2065. [Google Scholar]
- 41.Ozarowski A., Szymanska I.B., Muzio T., Jezierska J. J. Am. Chem. Soc. 2009;131:10279. doi: 10.1021/ja902695y. [DOI] [PubMed] [Google Scholar]
- 42.Clemente-Juan J.-M., Mackiewicz C., Verelst M., Dahan F., Bousseksou A., Sanakis Y., Tuchagues J.-P. Inorg. Chem. 2002;41:1478. doi: 10.1021/ic010787+. [DOI] [PubMed] [Google Scholar]
- 43.James F., Roos M. Comput. Phys. Commun. 1975;10:345. [Google Scholar]
- 44.Kato M., Muto Y. Coord. Chem. Rev. 1988;92:45. [Google Scholar]
- 45.Jagličić Z., Šegedin P., Zlatič J., Zorko A., Pirnat J., Trontelj Z. J. Magn. Magn. Mater. 2007;310:1444. [Google Scholar]
- 46.Dalai S., Mukherjee P.S., Zangrando E., Lloret F., Chaudhuri N.R. J. Chem. Soc., Dalton Trans. 2002:822. and references therein. [Google Scholar]
- 47.Knuuttila H. Inorg. Chim. Acta. 1983;69:173. [Google Scholar]
- 48.Clemente D.A. Inorg. Chim. Acta. 2005;358:1725. [Google Scholar]
- 49.Bonner J.C., Fisher M.E. Phys. Rev. A. 1964;135:640. [Google Scholar]
- 50.Estes W.E., Gavel D.P., Hatfield W.E., Hodgson D.J. Inorg. Chem. 1978;17:1415. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


